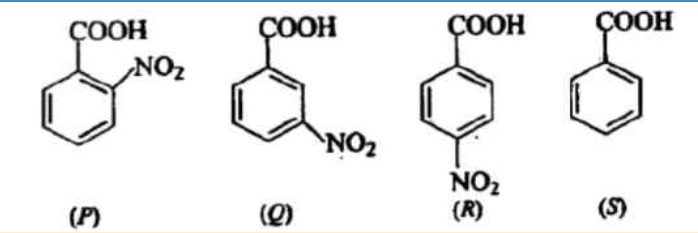
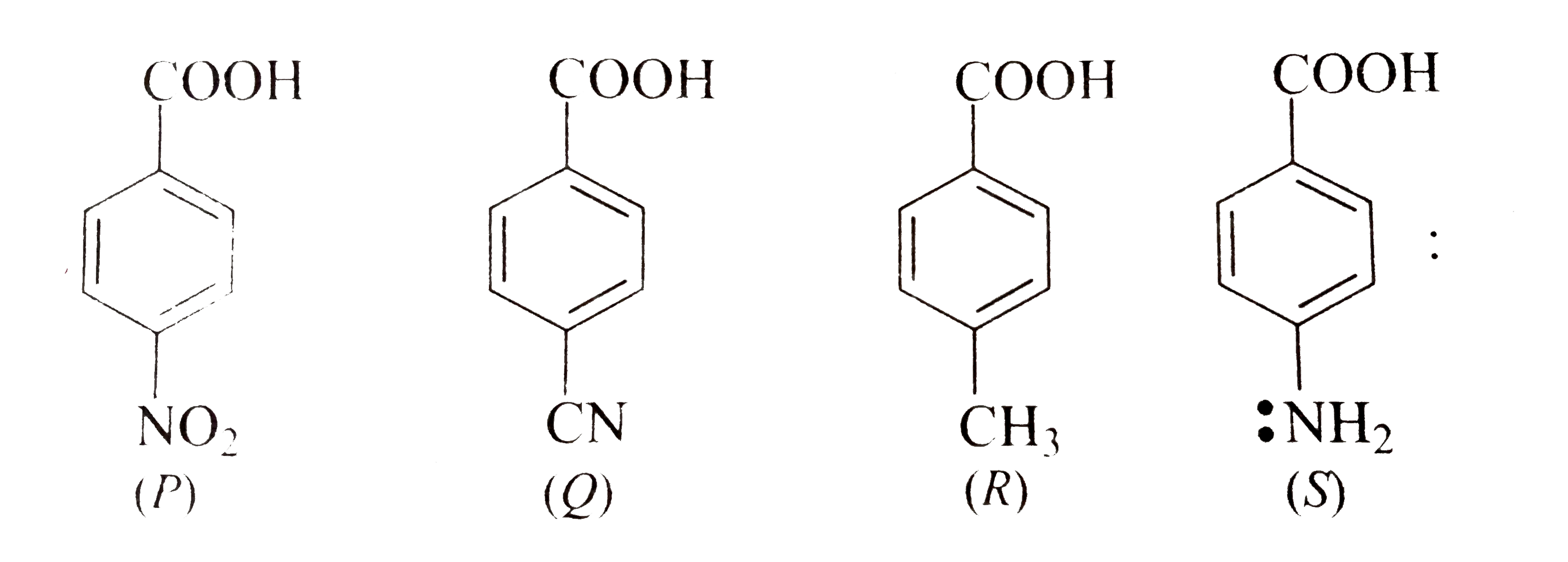
⇒ The decreasing order of acidity of following benzoic acid derivatives (Question No.101.108).
A
P>Q>R>S
B
Q>P>S>R
C
S>R>Q>P
D
R>S>P>Q
Video Solution
Text Solution
The correct Answer is:A
Withdrawing group will increase acidity while donating groups will decrease acidity.
|
Similar Questions
Knowledge Check
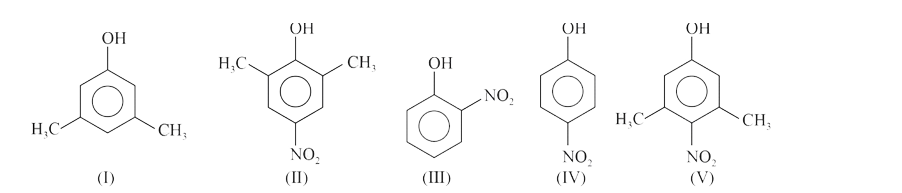
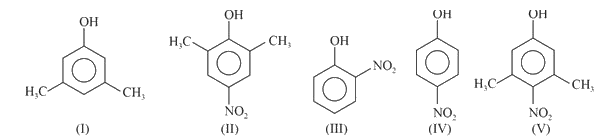
⇒ The decreasing order of acidity of following phenol derivatives is (Question No.93-100).
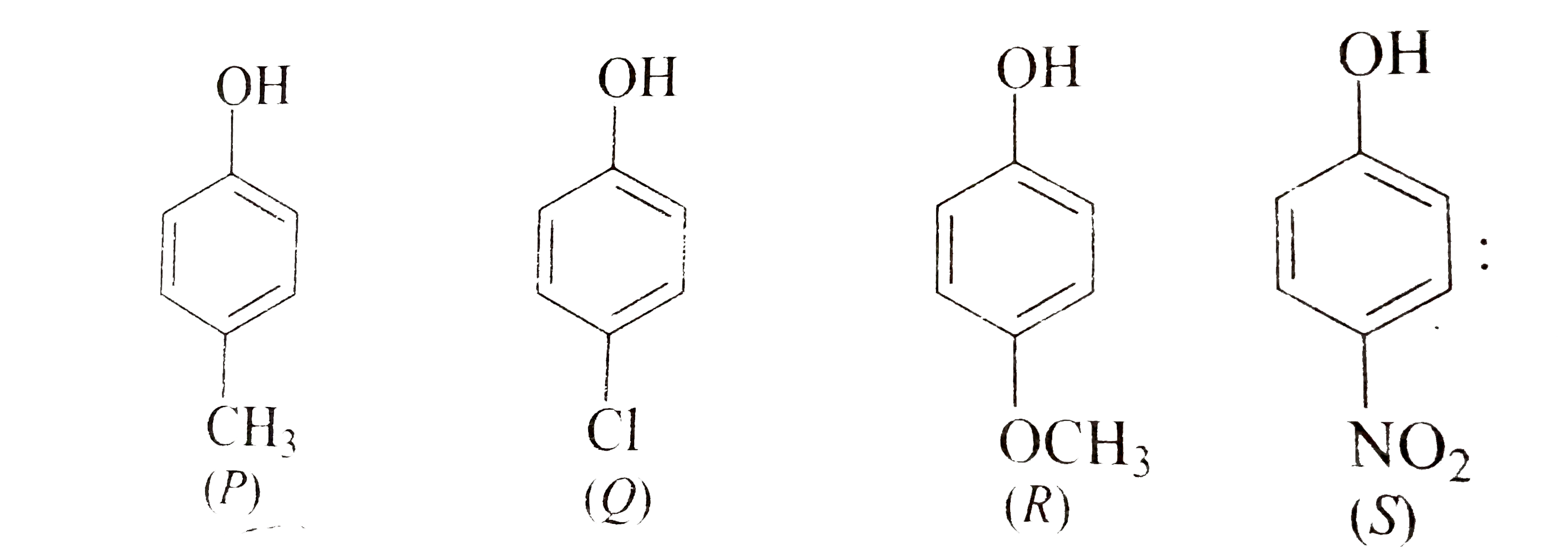 AS>P>Q>RBR>S>P>QCS>Q>P>RDP>Q>R>S
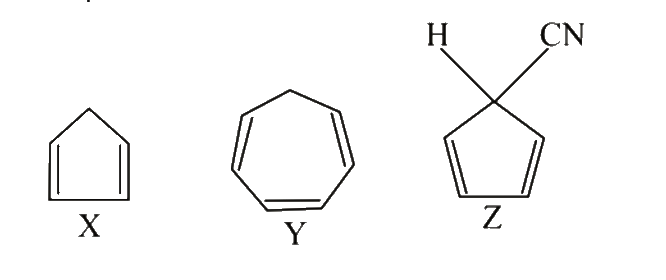
AS>P>Q>RBR>S>P>QCS>Q>P>RDP>Q>R>S⇒ The decreasing order of basicity of following aniline derivatives is (Question No.109-117).
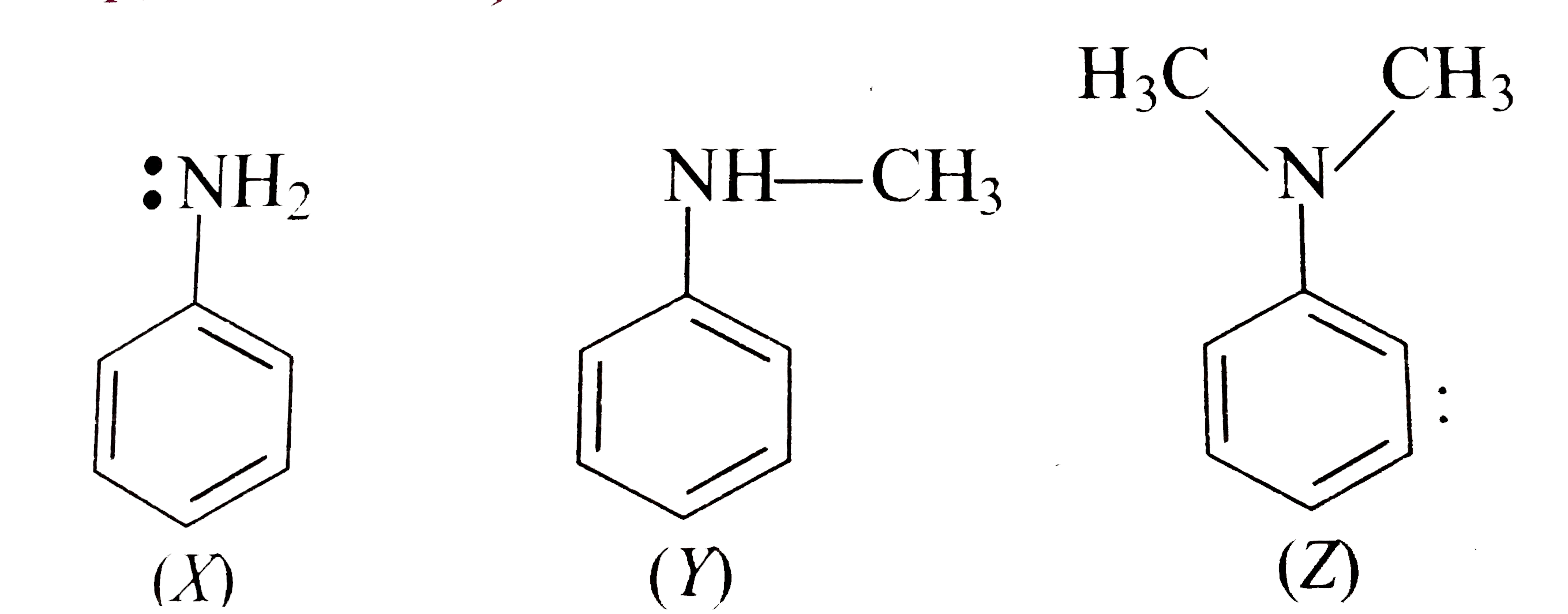 AX>Y>ZBY>Z>XCZ>Y>XDX>Z>Y
AX>Y>ZBY>Z>XCZ>Y>XDX>Z>YCorrect decreasing order of acidity of oxides is
ACl2O7>SO3>P4O10>SiO2BSO3>Cl2O7>P4O10>SiO2CSiO2>Cl2O7>SO3>P4O10DCl2O7>P4O10>SO3>SiO2
The decreasing order of acidic strength of the following is
View SolutionThe decreasing order of Acidity for the following compound will be

View SolutionDecreasing order of acidic strength of following compound is
View SolutionThe decreasing order of acidic strength of the following is:
View SolutionThe decreasing order of acidic character is :
View Solution