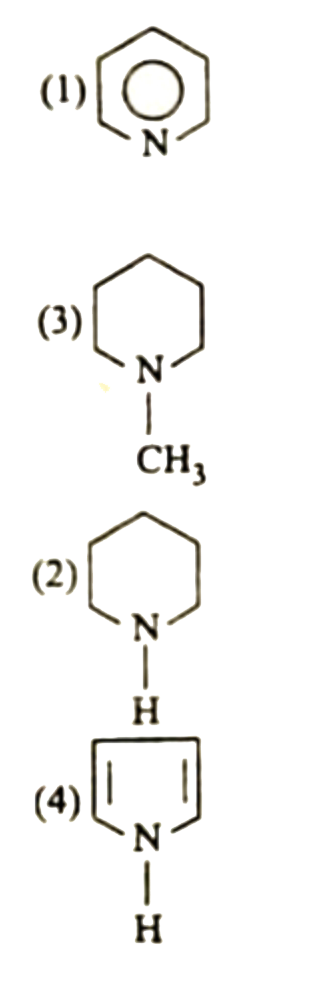
⇒ Arrange the following nitrogen containing compounds in decreasing order of basicity (Question No.126-130).
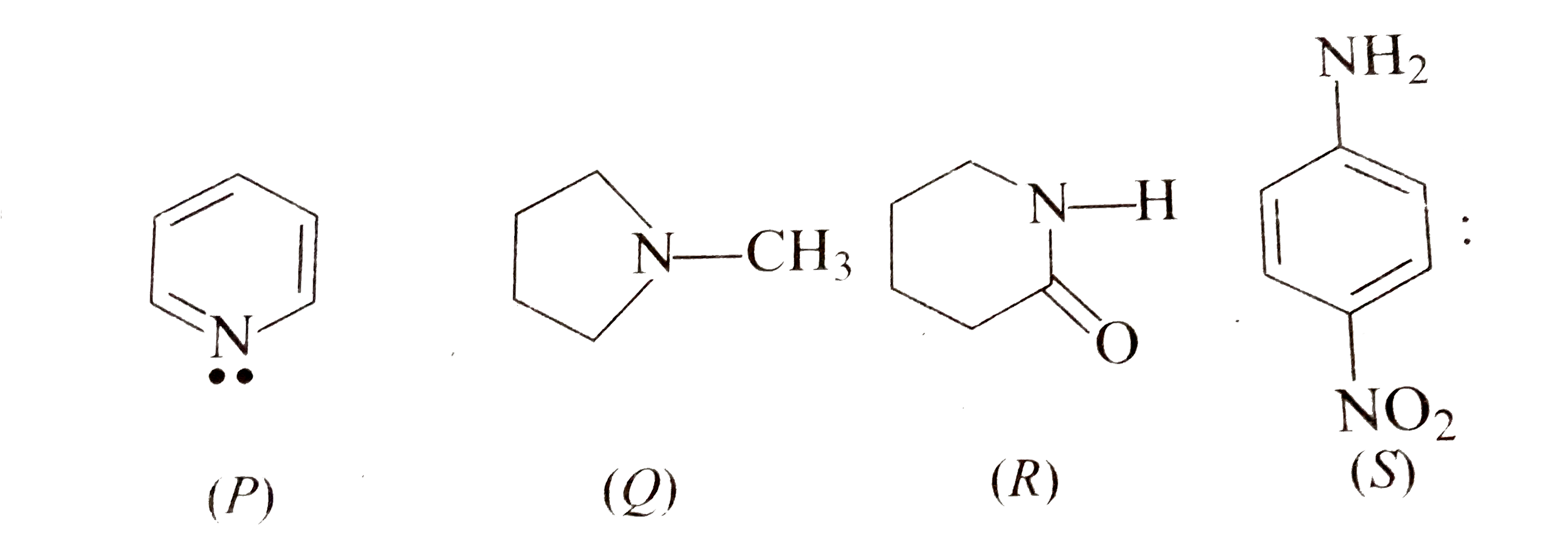
A
P>Q>R>S
B
Q>P>R>S
C
Q>P>S>R
D
S>P>Q>R
Video Solution
Text Solution
The correct Answer is:B
Q is most basic as it is aliphatic amine, P is more basic than R and S as lpe− of P is delocalise.
|
Similar Questions
Knowledge Check
Arrange the following compounds in decreasing order of basicity :
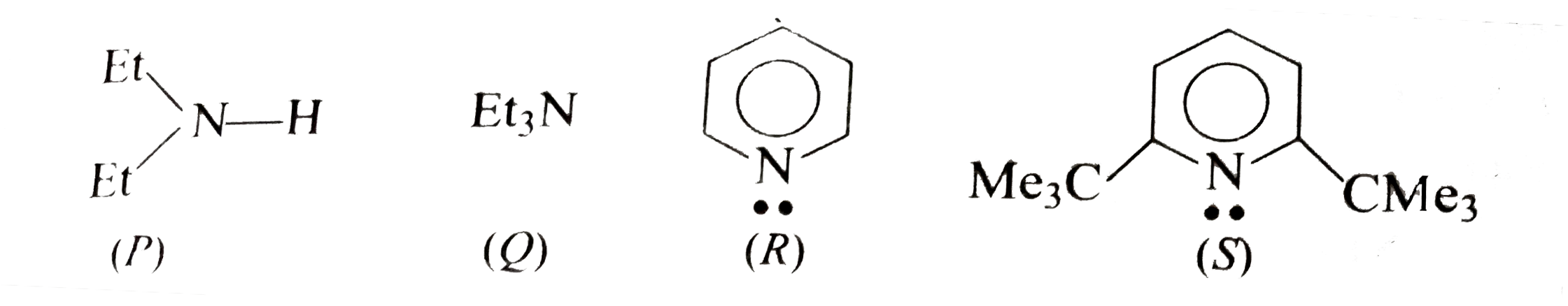 AP>Q>R>SBQ>P>R>SCR>S>Q>PDQ>P>S>R
AP>Q>R>SBQ>P>R>SCR>S>Q>PDQ>P>S>RArrange the following compounds in increasing order of basicity :
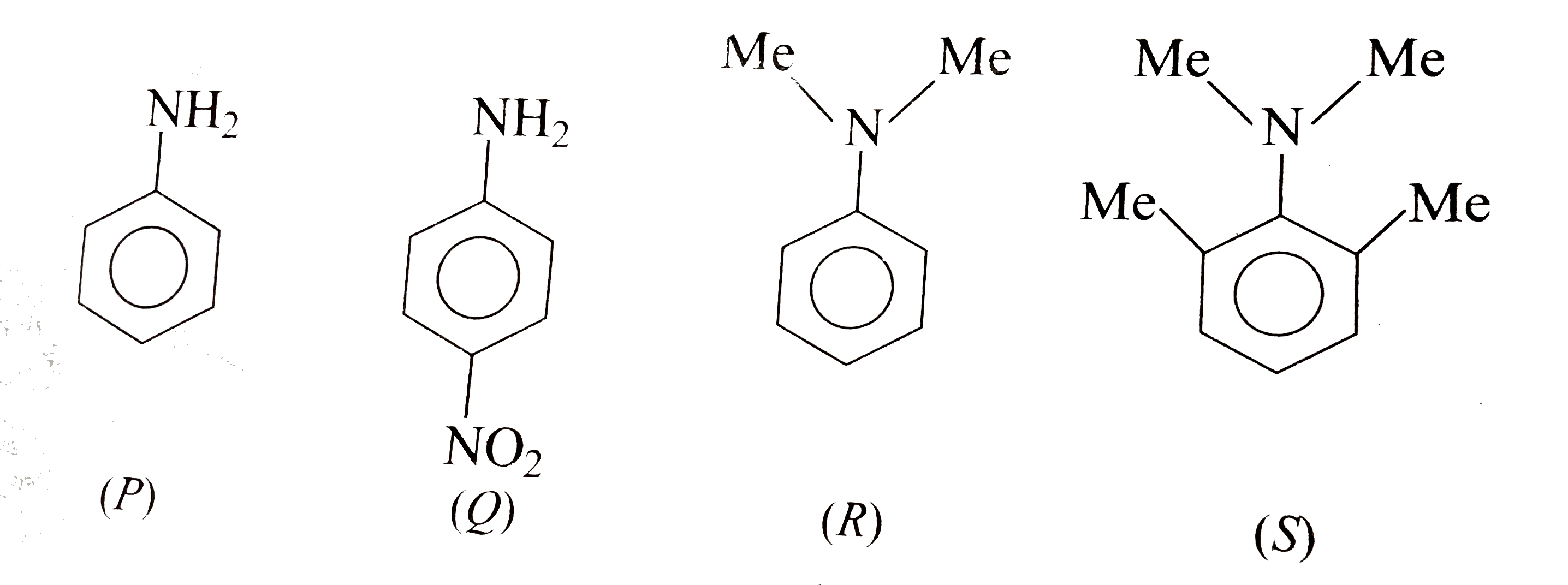 AS>R>Q>PBS>R>P>QCP>Q>R>SDR>Q>P>S
AS>R>Q>PBS>R>P>QCP>Q>R>SDR>Q>P>SArrange the following compounds in decreasing order of their heats of combustion:
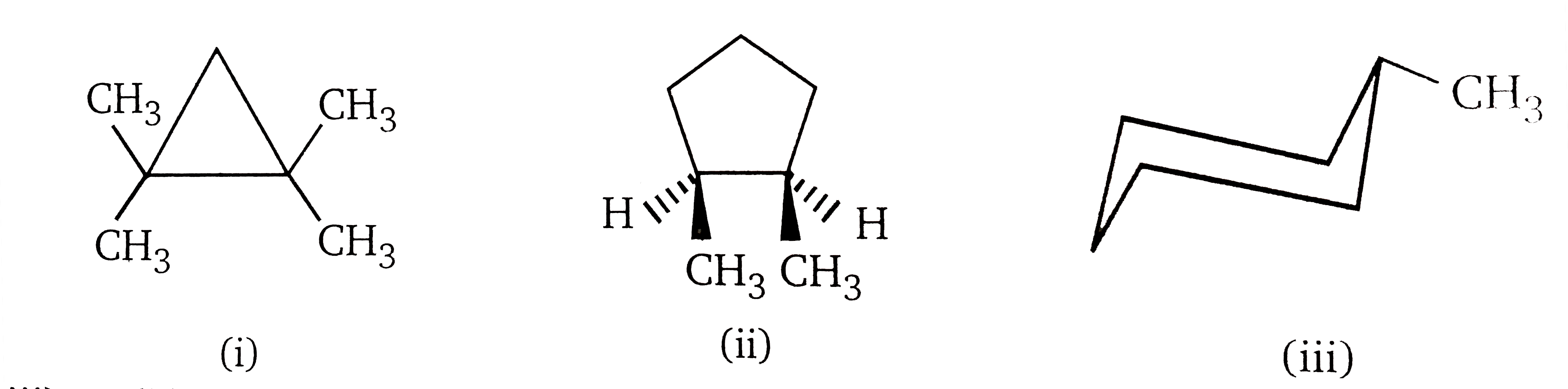 A(ii)>(ii)>(i)B(ii)>(i)>(iii)C(iii)>(i)>(ii)D(i)>(ii)>(iii)
A(ii)>(ii)>(i)B(ii)>(i)>(iii)C(iii)>(i)>(ii)D(i)>(ii)>(iii)
Arrange the following in decreasing order of basicity:

View SolutionArrange the following compounds in decreasing order of acidity.
H2O,ROH,HC≡CH
View SolutionArrange the following compounds in decreasing order of their acidic strength.

View SolutionArrange basicity of given compounds in decreasing order
View SolutionArrange basicity of given compounds in decreasing order
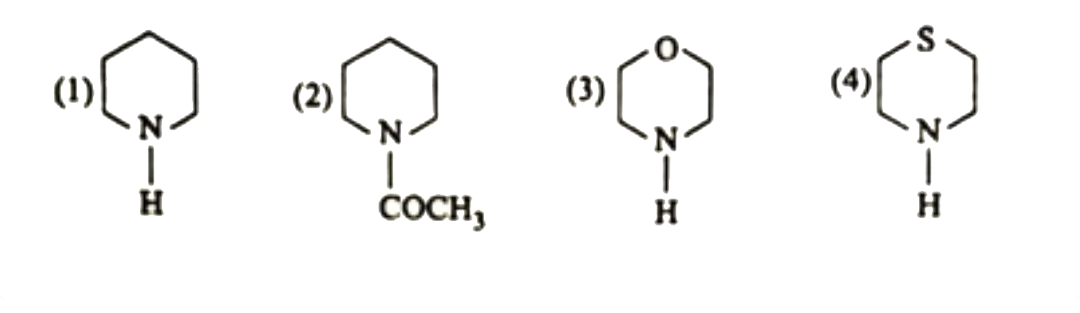
View Solution

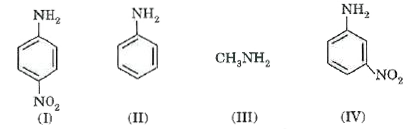
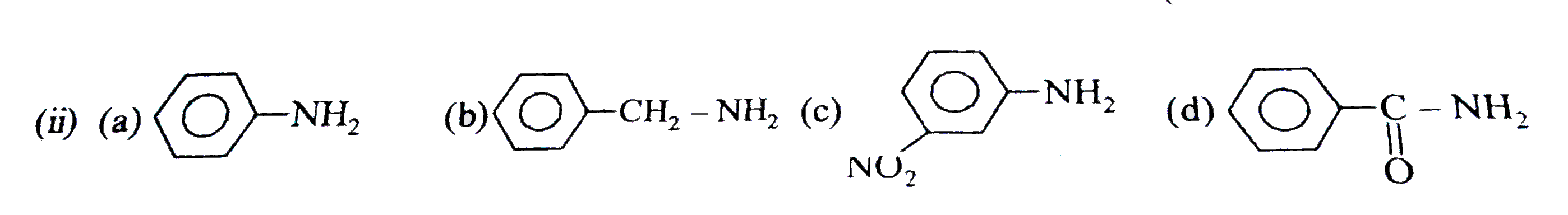 .
.