Which of the following orders is correct order of these anions?
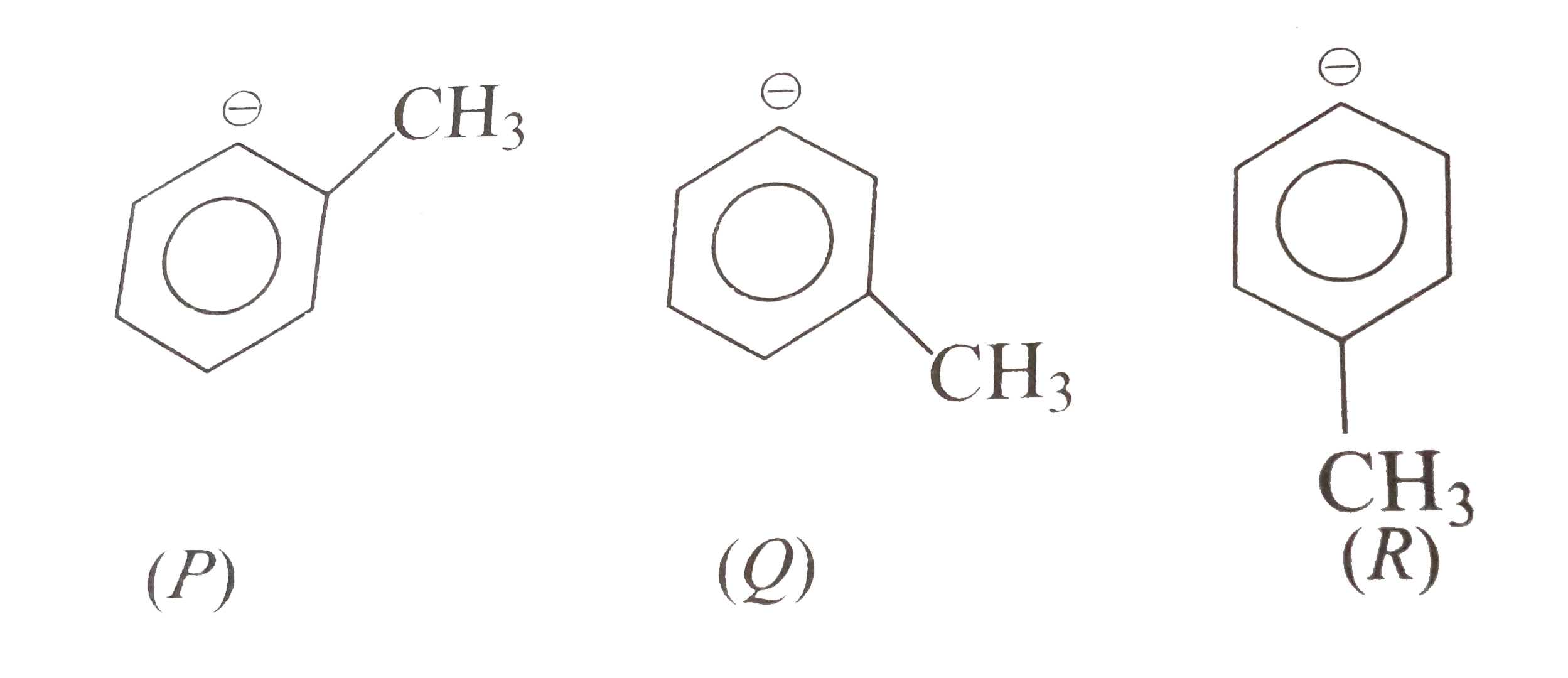
A
Q>R>P
B
Q>P>R
C
R>Q>P
D
P>Q>R
Video Solution
Text Solution
The correct Answer is:A
P and R are less stabilise because of hyperconjugtion.
|
Similar Questions
Knowledge Check
Which of the following order is correct ?
AB<C<N<O (First ionisation energy)BLi<Na<Rb<K (size)CCI>F>Br>I (Magnitude of ΔHeg.)DF<O<N<C (Electronegativity)Which of the following order is correct ?
AK+<Ca2+<P3−<S2− : Ionic sizeBNa+(aq.)>K+(aq.)>Rb+(aq.)>Cs+(aq.) Electrical conductanceCAl3+(aq.)>Mg2+(aq)>Na+(aq.) , Hydrated sizeDI−(aq.)<Br−(aq.)<Cl−(aq.)<F−(aq.) : Ionic mobilityWhich of the following order is correct?
AK+<Ca2+<P3−<S2− Ionic sizeBNa+aq.>K+aq.>Rb+aq.>Cs+aq.: Electrical conductanceCAl3+aq.>Mg2+aq.>Na+aq.: Hydrated sizeDI−aq.<Br−aq.>Cl−aq.>F−aq.: Ionic mobility
