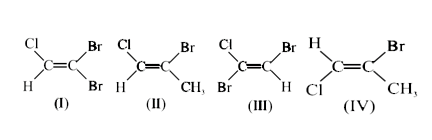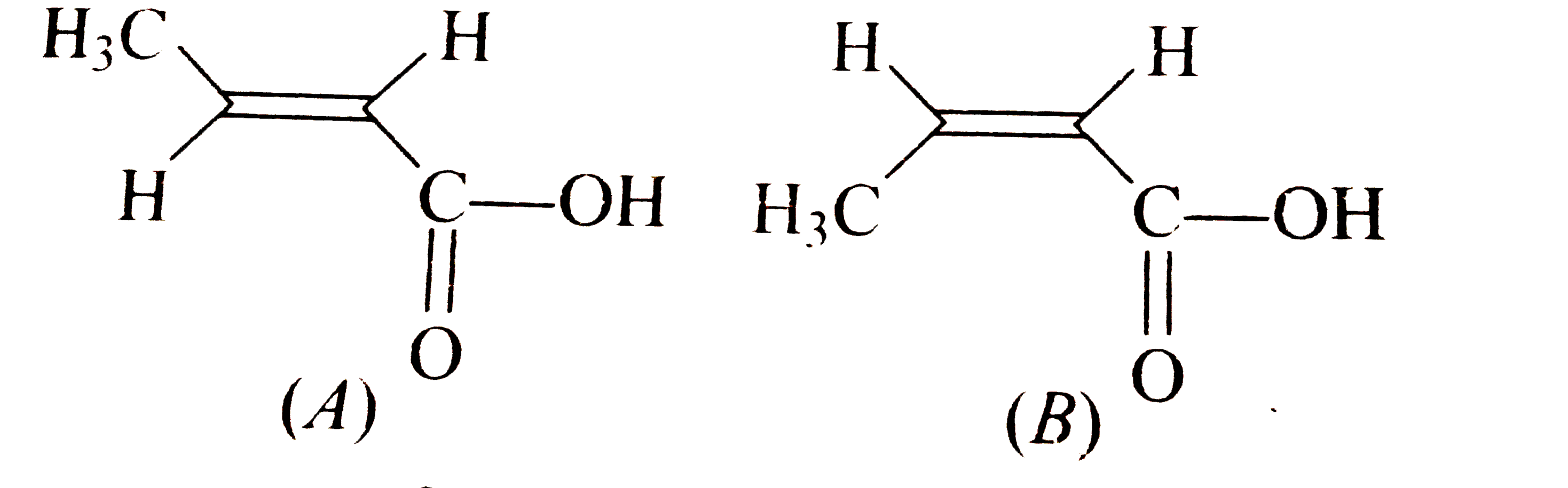
Which of the following statement are correct about this pair of geometrical isomer?
A
−I effect of vinylic group on the −COOH group is same in both isomers. So there is no role of −I on the strength of acidity
B
+M power of vinylic group is operating effectivel in trans isomer (A) but not in cis-isomer (B) because of steric repulsion in cis-isomer causing loss of coplanarity of −COOH gp. With C=C bond . Therefore, cis-isomer is more acidec than trans
C
+I power of CH3− is greater in trans than cis
D
None of the above
Text Solution
The correct Answer is:A, B
|
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRYBook:HIMANSHU PANDEYChapter:GENERAL ORGANIC CHEMISTRYExercise:More Than One Correct (Q.51 To Q.55)
Explore 5 Videos
- GENERAL ORGANIC CHEMISTRYBook:HIMANSHU PANDEYChapter:GENERAL ORGANIC CHEMISTRYExercise:Linked Comprehension Type (Q.1 To Q.25)
Explore 25 Videos
- GENERAL ORGANIC CHEMISTRYBook:HIMANSHU PANDEYChapter:GENERAL ORGANIC CHEMISTRYExercise:More Than One Correct (Q.1 To Q.25)
Explore 25 Videos
- CHEMISTRY IN DAILY LIFEBook:HIMANSHU PANDEYChapter:CHEMISTRY IN DAILY LIFEExercise:Integer Answer Type Problems
Explore 7 Videos
- HALIDESBook:HIMANSHU PANDEYChapter:HALIDESExercise:Subjective Type Problems
Explore 10 Videos
Similar Questions
Knowledge Check
Which of the following statements are correct about halr-period ?
AIt is proportional to intial concentration for zeroth orderBAverage life =1.44× half-life for first order reaction.CTiem of 75% comletion of reaction is thrice of halr-life (intial harl-life) period in second order reactionD99.9% reaction takes place in 100 minutes for the case when reate constant is 0.693−1min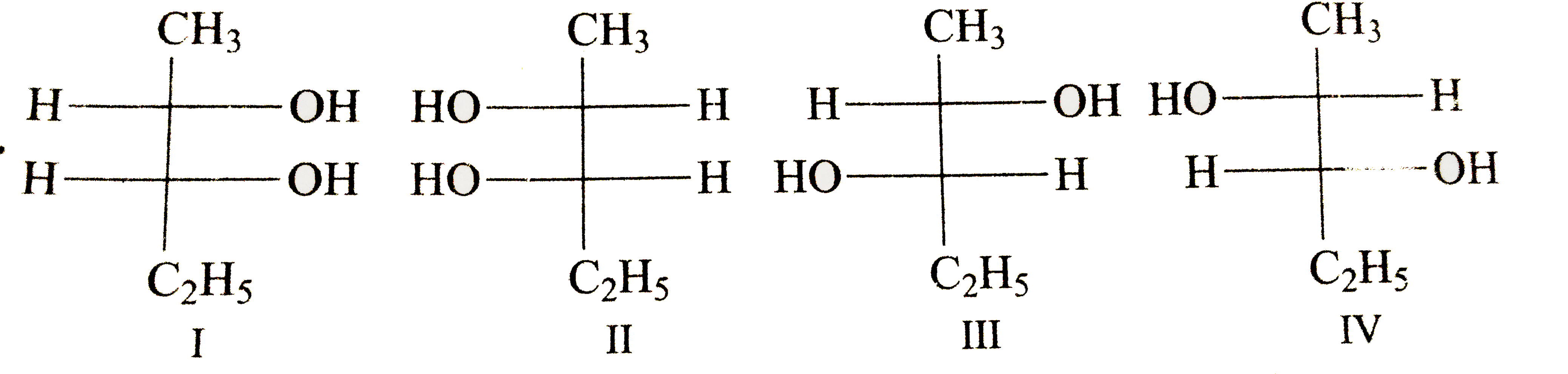
Which of the following statements are true about these isomers?AI and II are pair of enantiomersBIII and IV are a pair of enantiomersCII is the diastereomer of III and IVDI and III are homomersWhich of the following statement is correct about metaboric acid?
AIt has one hydrogen which is attached with boron atom.BBoron has covalency '5'.COrthoboric acid is produced on addition of one mole H2O to one mole metaboric acid.DMetaboric acid is not an oxy-acid.