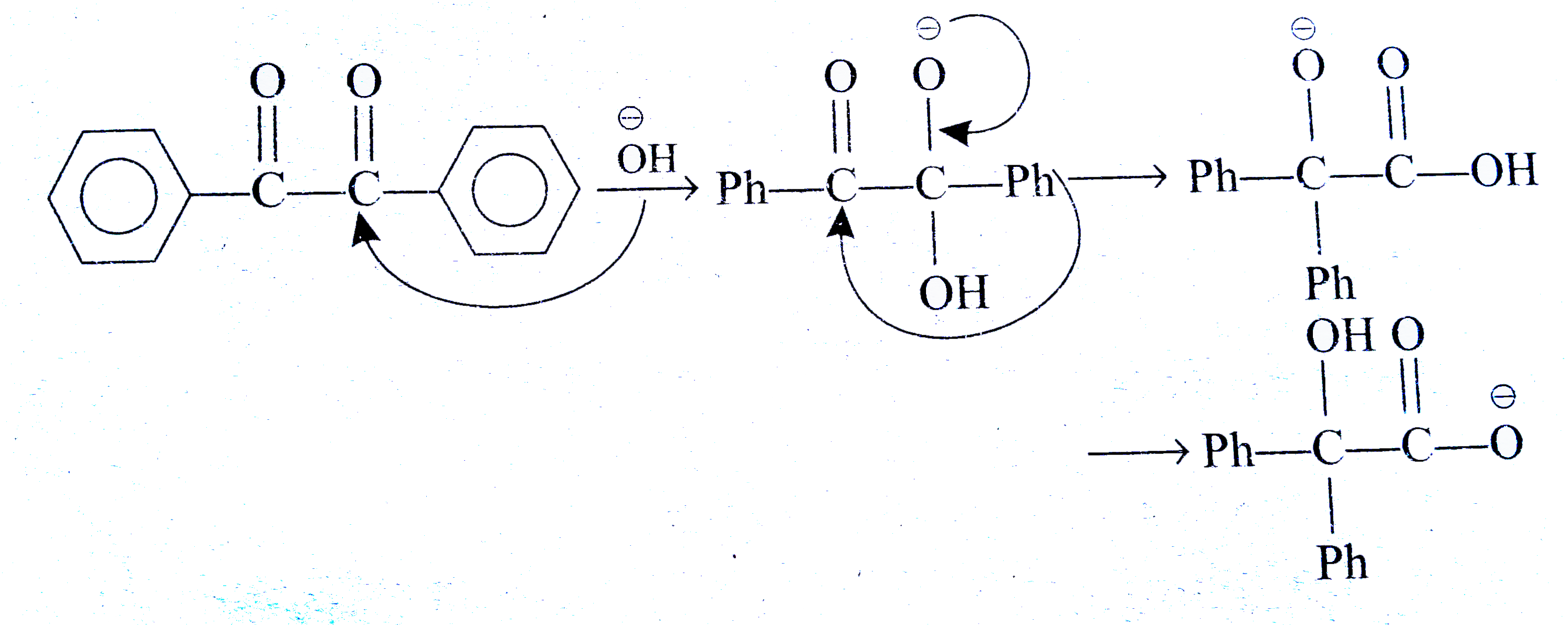
The base catalysed reaction of 1,2-diketone to a salt of 2-hydroxy carbonxylic acid is known as benzile acid rearrangement . The reaction is not application to 1,2-diketones containing α− methylene group because of competing aldol condensation .
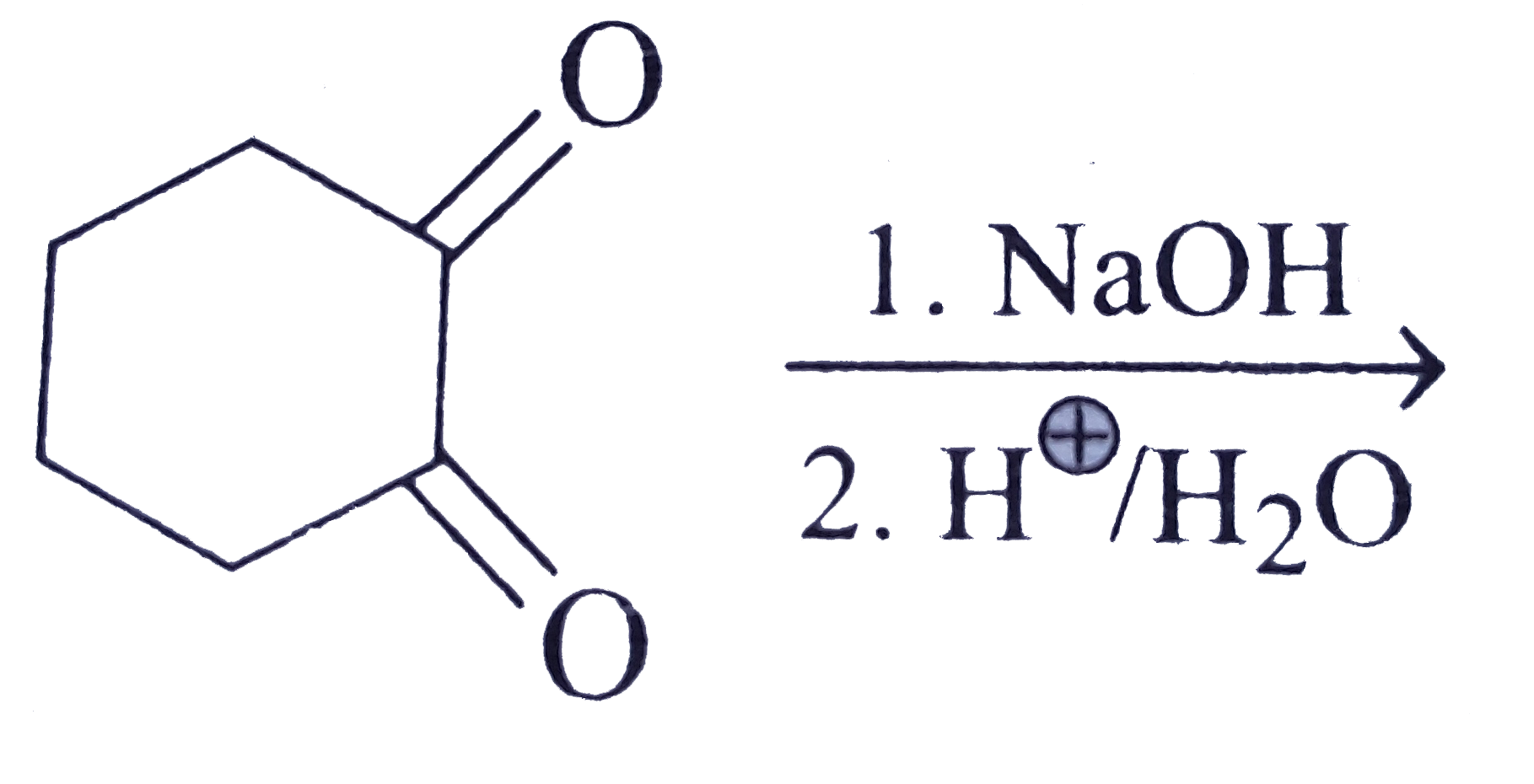
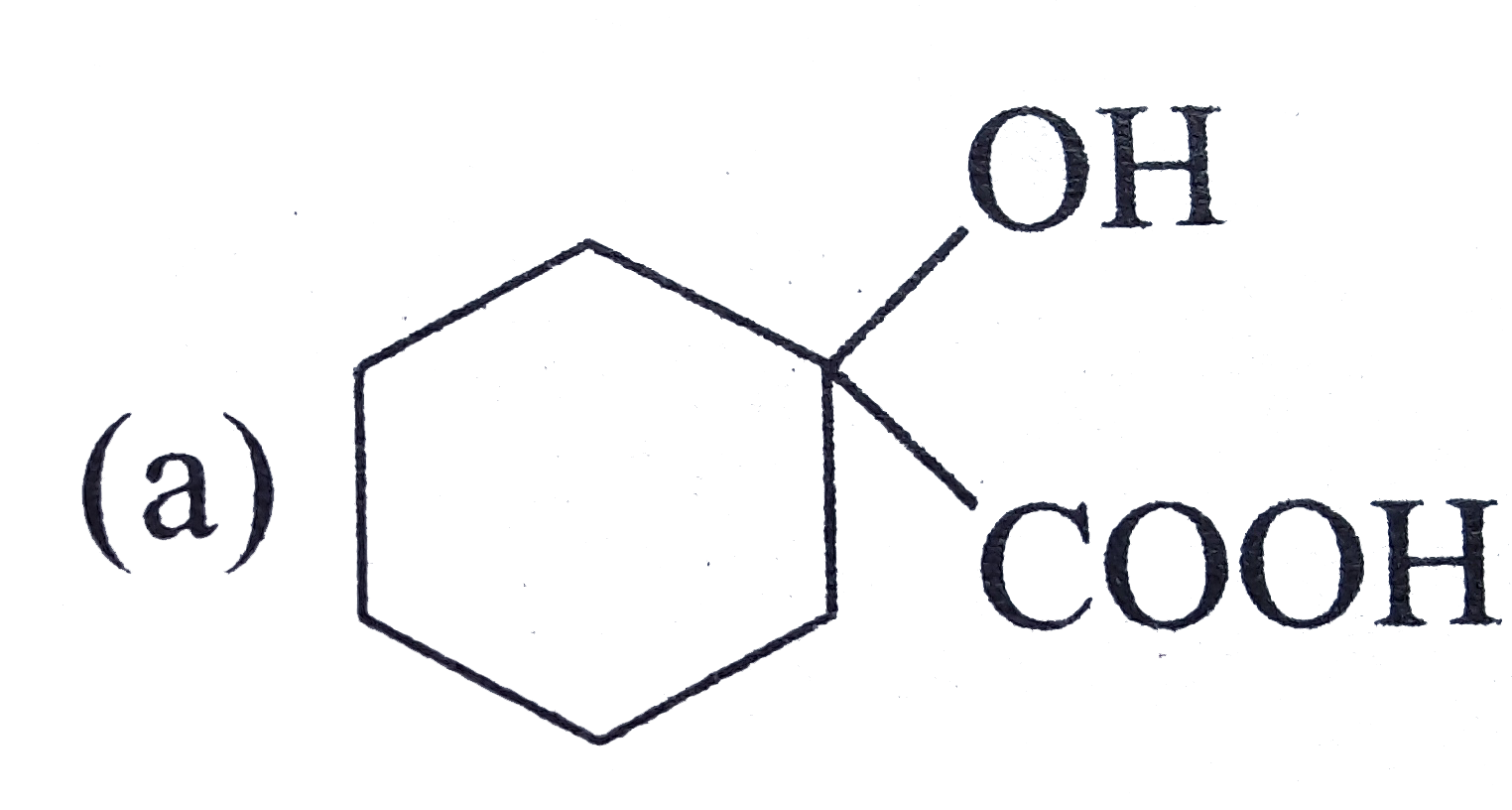
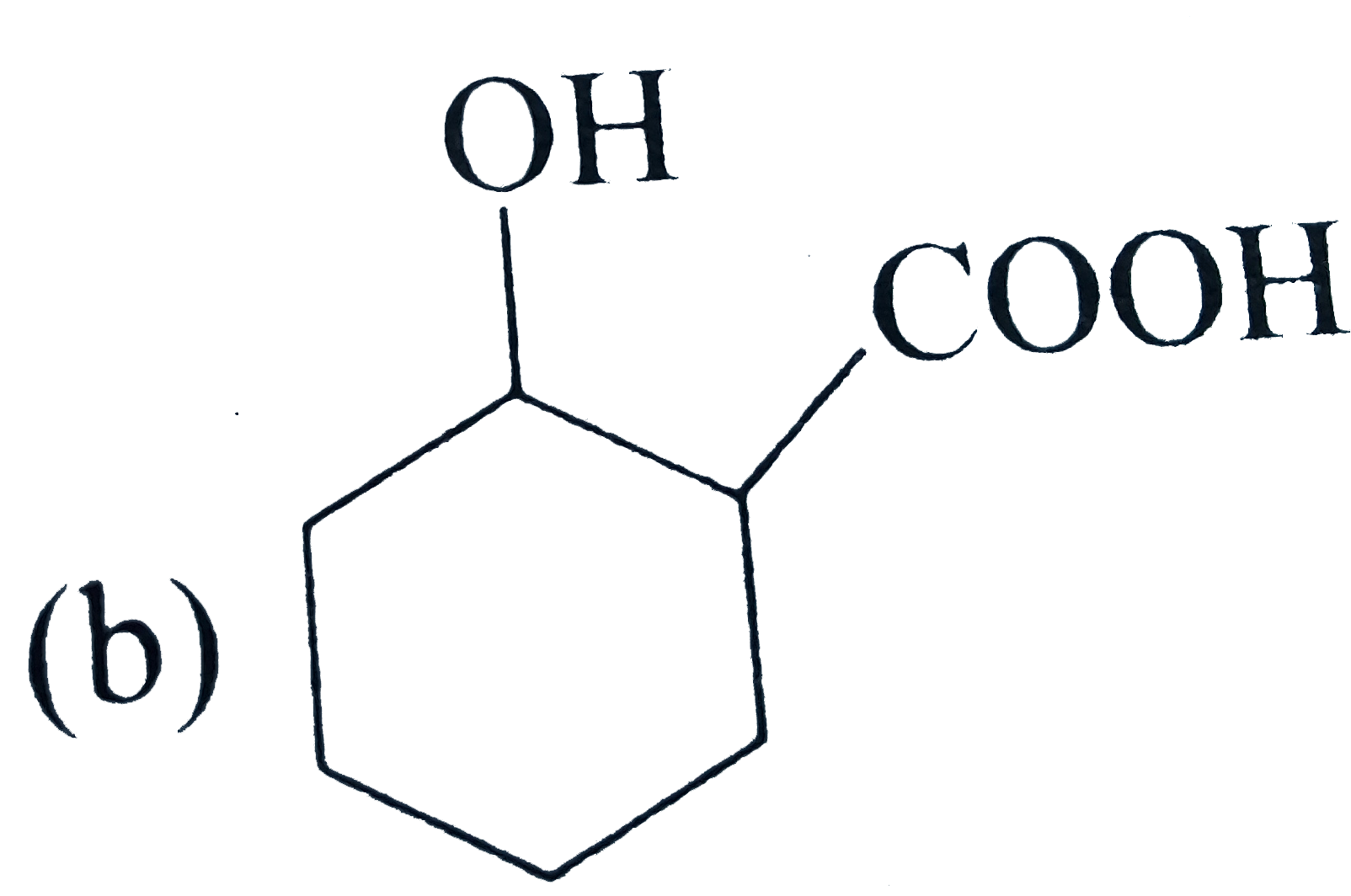
The correct Answer is:C
Step by step video, text & image solution for The base catalysed reaction of 1,2-diketone to a salt of 2-hydroxy carbonxylic acid is known as benzile acid rearrangement . The reaction is not application to 1,2-diketones containing alpha- methylene group because of competing aldol condensation . by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Benzoin condensation Reaction|| Benzil-Benzilic acid Rearrangement||Reformatsky Reaction||Reaction OF active methylene group|| Knoevenagel Reaction|| Michael addition Reaction
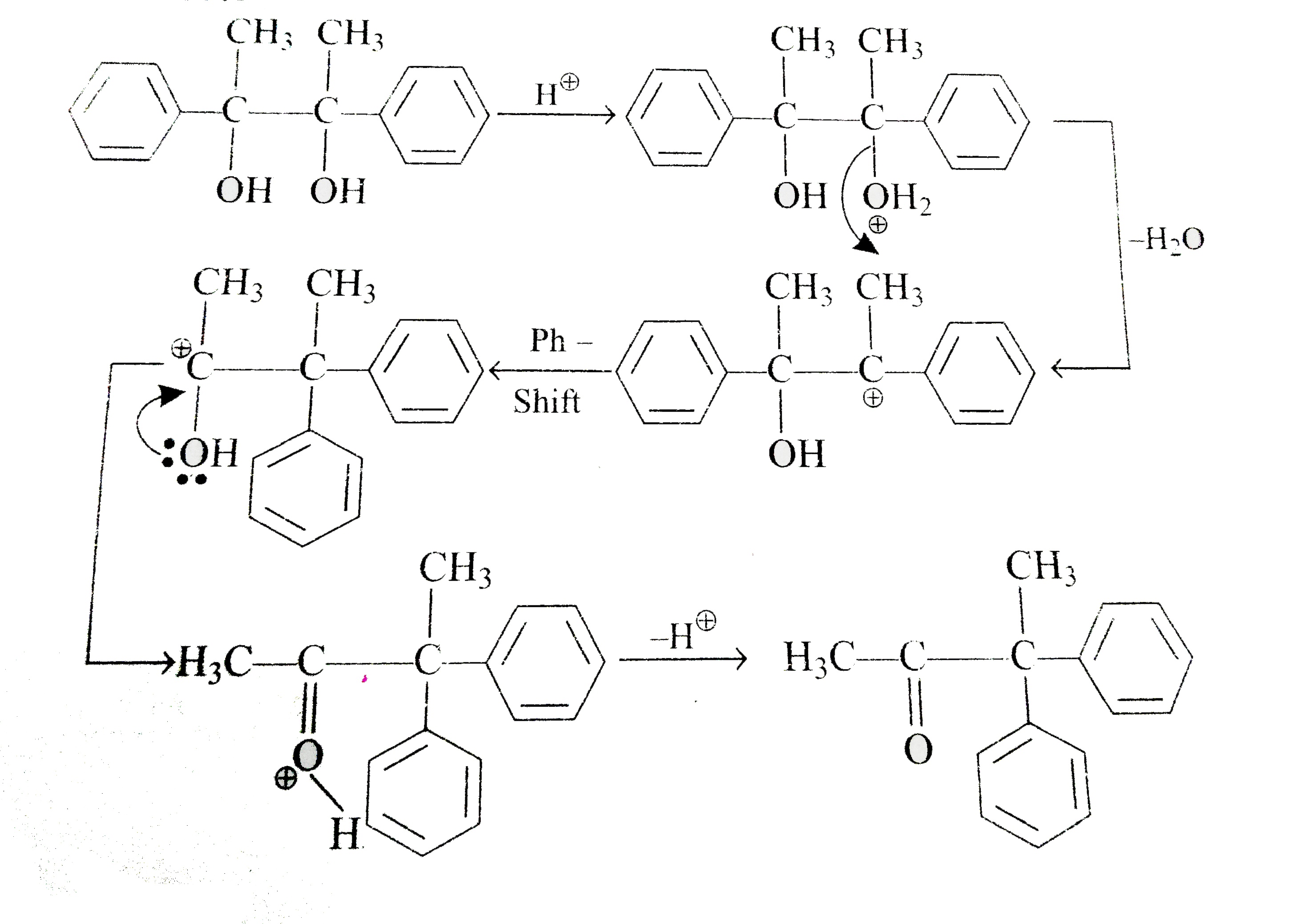
View SolutionAcid catalysed conversation of 1,2-diol or vicinal, into carbonyl compound known as pinocaol-pinacolone rearrangement.

Generally more electron donating group migrate during mechanism, migration of H is faster because of its smaller size.
What would be the major product of reaction?
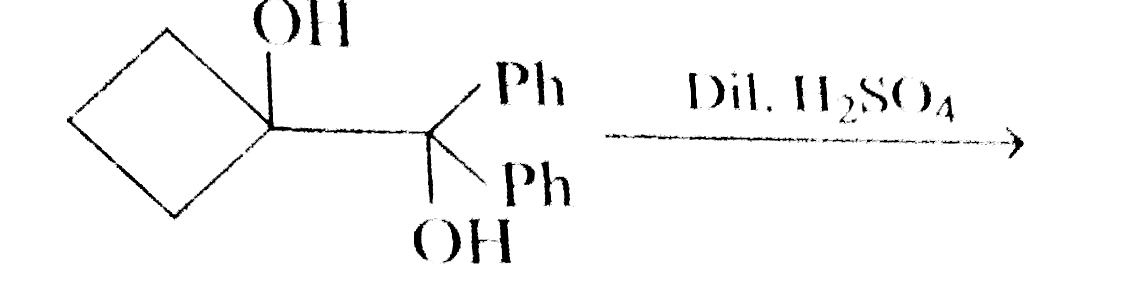
View Solution
Knowledge Check
Common name of Ethane -1,2- dioic acid is known as
AOxalic acidBPhthalic acidCAdipic acidDAcetic acidAcid catalysed conversation of 1,2-diol or vicinal, into carbonyl compound known as pinocaol-pinacolone rearrangement.
Generally more electron donating group migrate during mechanism, migration of H is faster because of its smaller size.
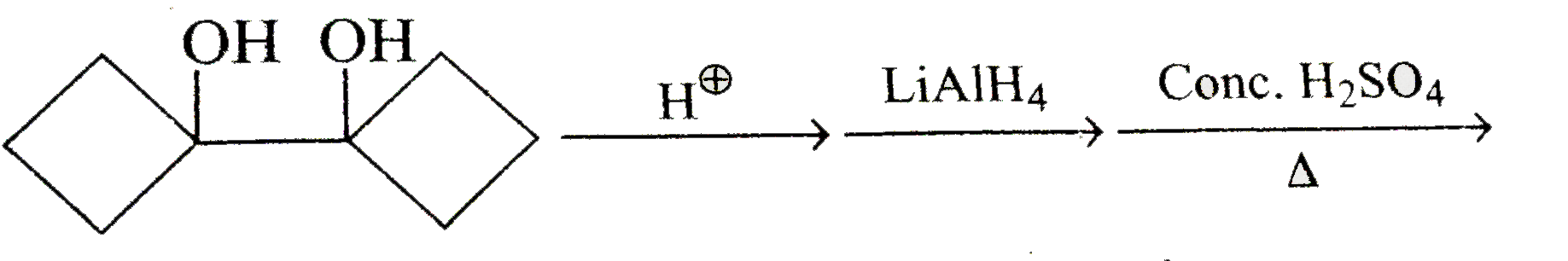
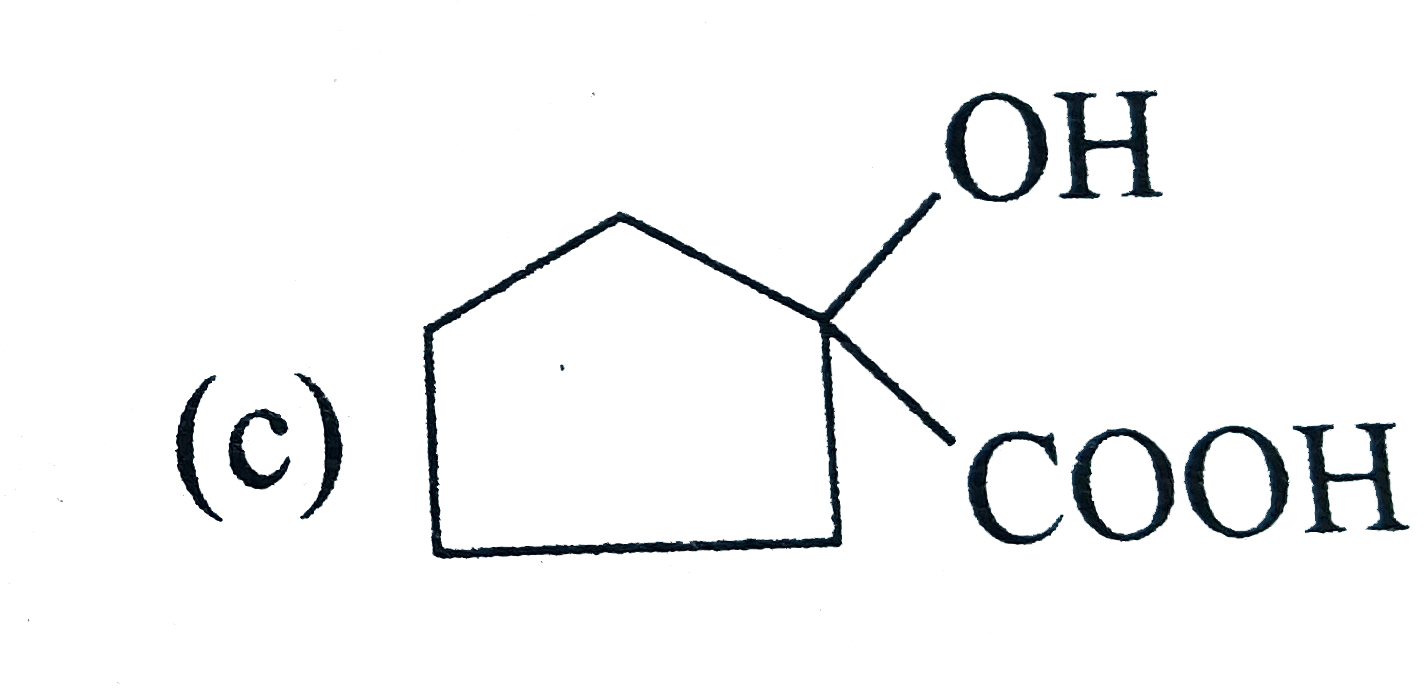
In this sequence of reaction final product is"A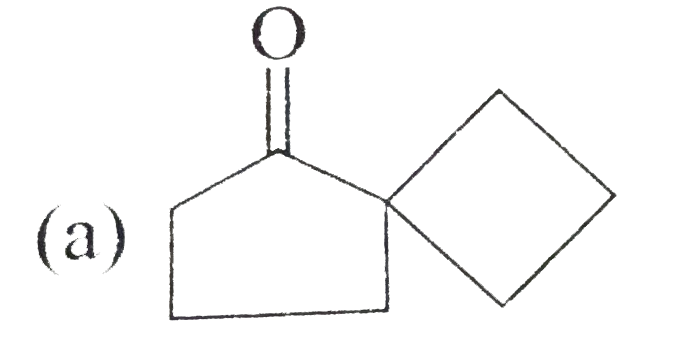 B
B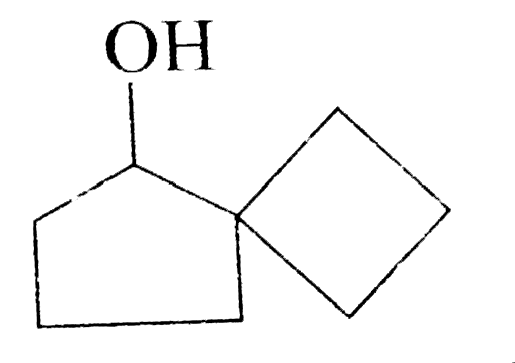 C
C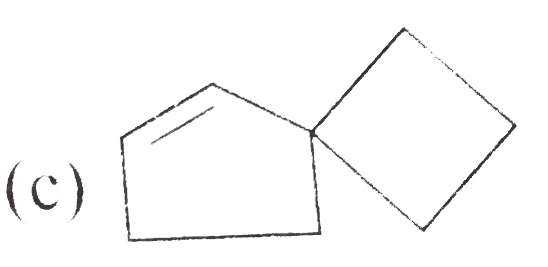 D
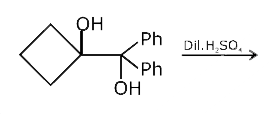
D
Acid catalysed conversion of 1,2-diol or vicinal diol, into carbonyl compound known as pinacol-pinacolone rearrangement.

Generally more electron donating group migrate during mechanism, migration of –H is faster because of its smaller size.
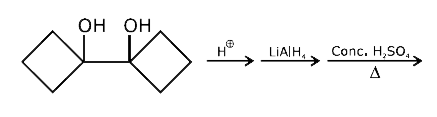
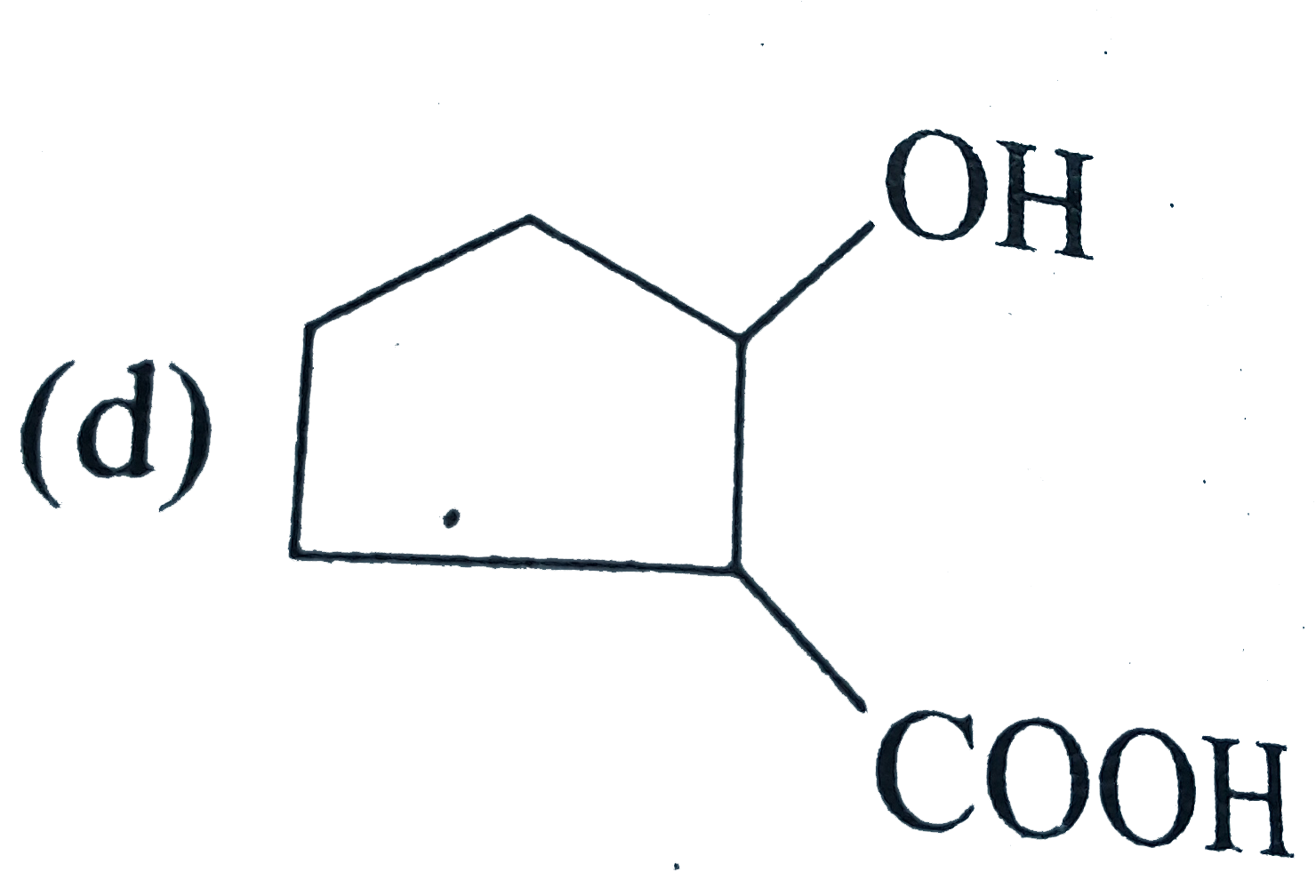
In this sequence of reaction final product isA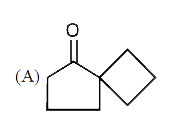 B
B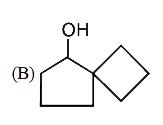 C
C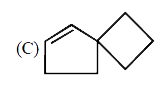 D
D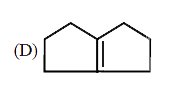
Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl compound known as pinocaol-pinacolone rearrangement.

Generally more electron donating group migrate during mechanism, migration of H is faster because of its smaller size.
Which of the following is not correct about this rearrangement?
View SolutionAcid catalysed conversation of 1,2-diol or vicinal, into carbonyl compound known as pinocaol-pinacolone rearrangement.

Generally more electron donating group migrate during mechanism, migration of H is faster because of its smaller size.
Which of the following is not correct about this rearrangement?
View SolutionThe base catalysed addition of a compound having active methylene group (or relatively acidic hydrogen) to activate alkene is known as Michael addition reaction.



View SolutionThe base catalysed addition of a compound having active methylene group (or relatively acidic hydrogen) to activate alkene is known as Michael addition reaction.


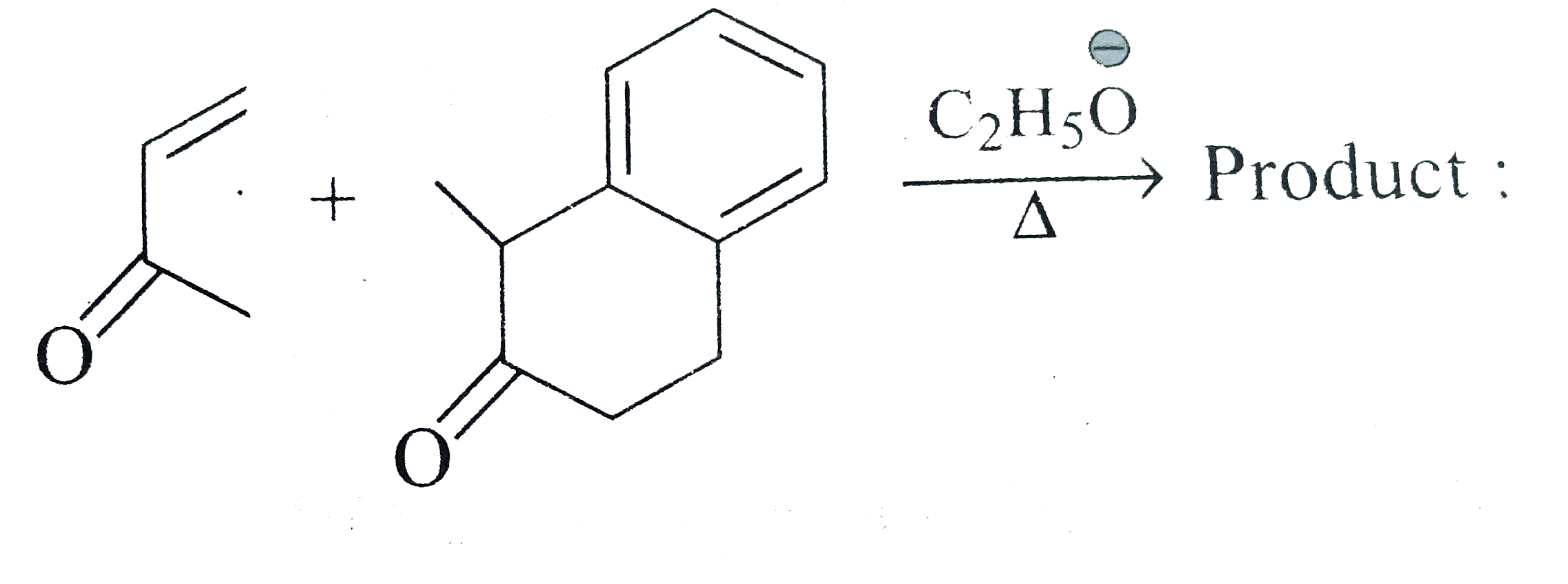
View SolutionSilver salt of 2-methyl propanoic acid on reaction with bromine gives
View Solution
