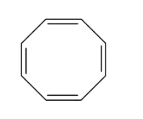
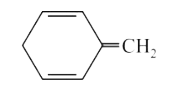
Explain why the following systems are not aromatic? 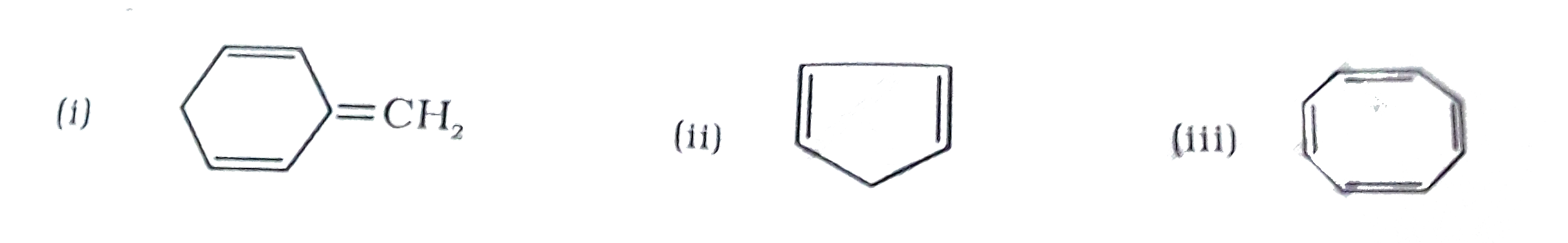
Video Solution
Text Solution
The correct Answer is:Lack of delocalisation of (4n+2)π electrons in the cyclic system.
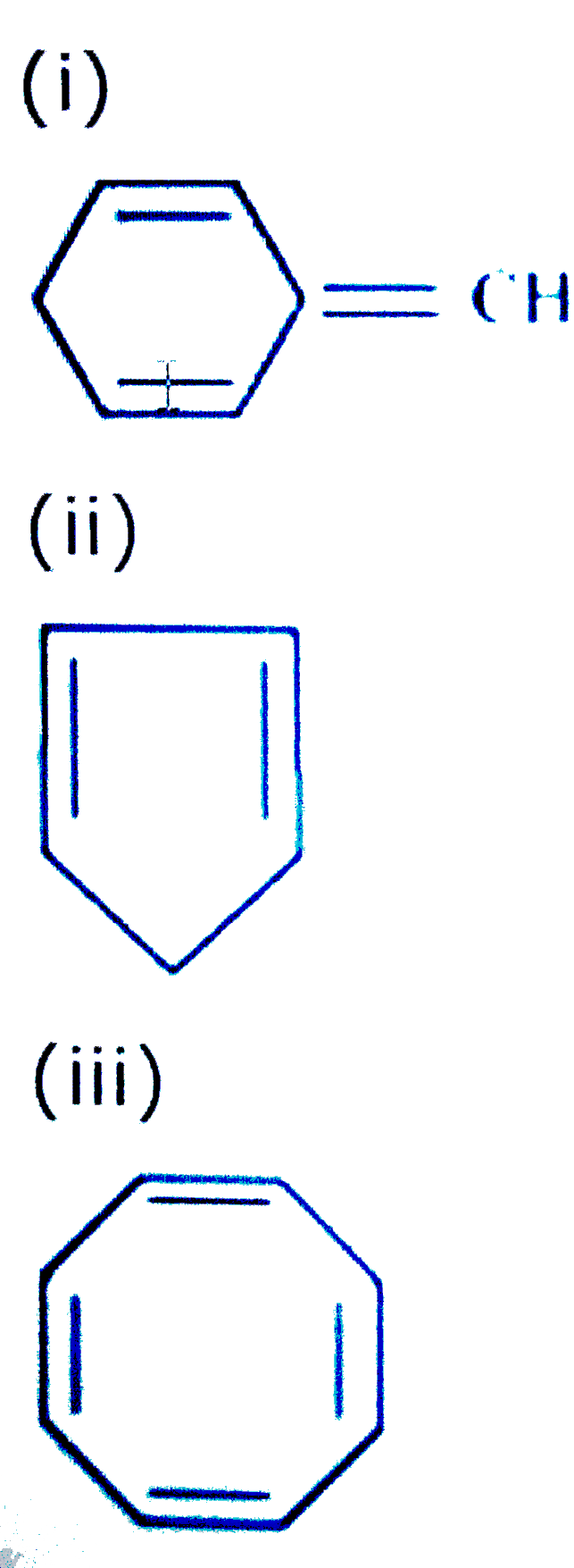 For the given compound, the number of (π)-electrons is 6. By Huckel’s rule,
For the given compound, the number of (π)-electrons is 6. By Huckel’s rule,
4n + 2 = 6
4n = 4
n = 1
For a compound to be aromatic, the value of n must be an integer (n = 0, 1, 2…). Since the value of n is an integer, the given compound is aromatic in nature.
(ii) 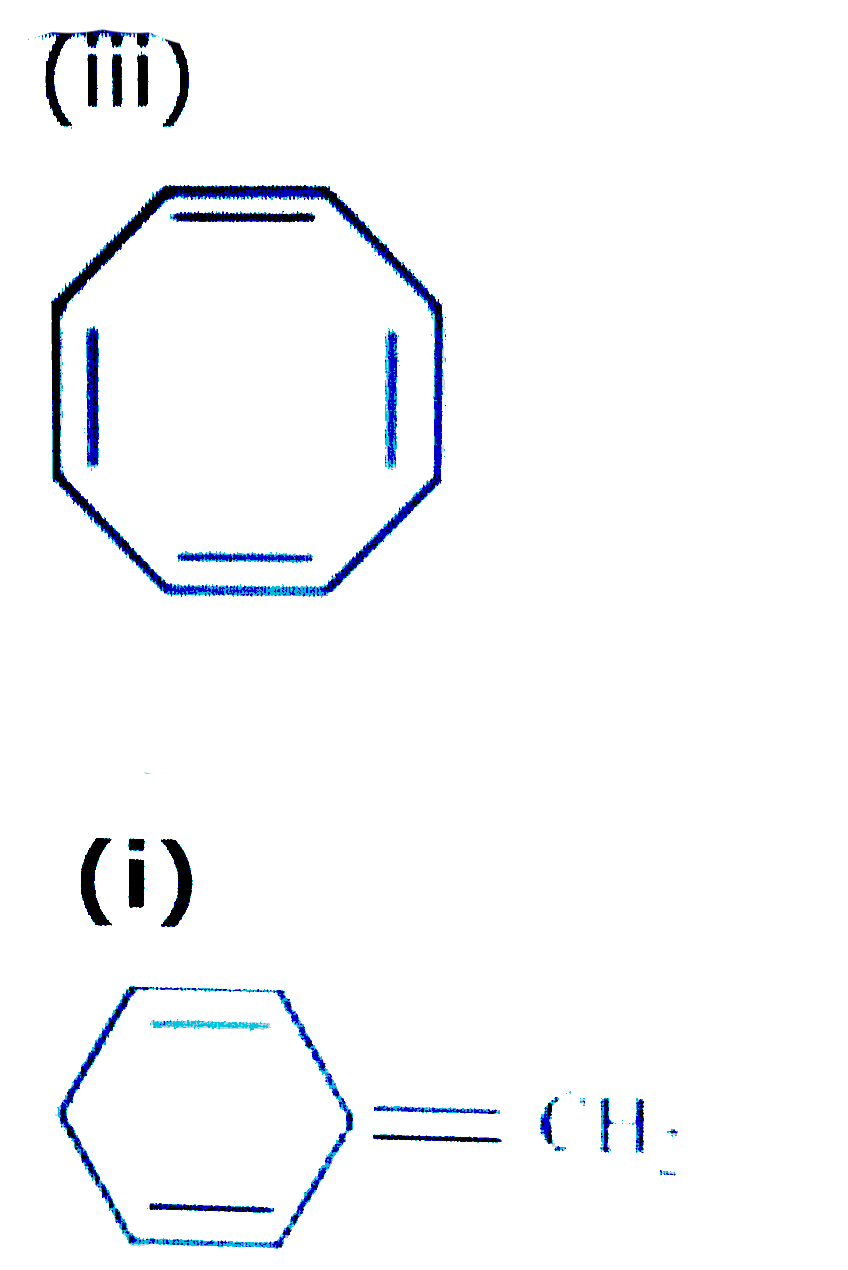 For the given compound, the number of π-electrons is 4. By Huckel’s rule,
For the given compound, the number of π-electrons is 4. By Huckel’s rule,
4n + 2 = 4
4n=2
n=12
For a compound to be aromatic, the value of n must be an integer (n = 0, 1, 2…), which is not true for the given compound. Hence, it is not aromatic in nature.
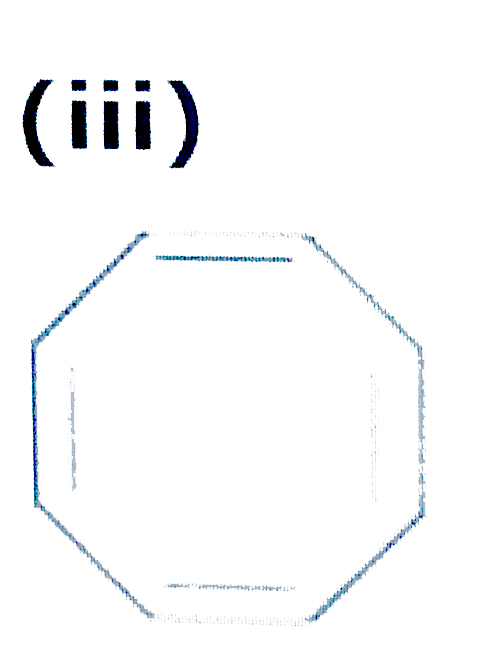
 For the given compound, the number of π-electrons is 8.
For the given compound, the number of π-electrons is 8.
By Huckel’s rule, 4n+2=8
4n=6
n=32
For a compound to be aromatic, the value of n must be an integer (n = 0, 1, 2…). Since the value of n is not an integer, the given compound is not aromatic in nature.
|
Similar Questions
Knowledge Check
Which of the following are not aromatic?
A B
B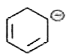 C
C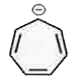 D
D
Which of the following is not aromatic?
A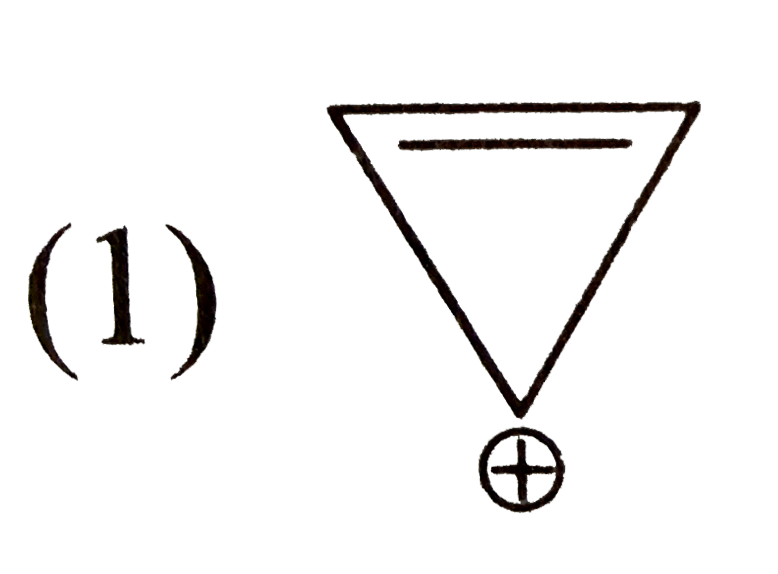 B
B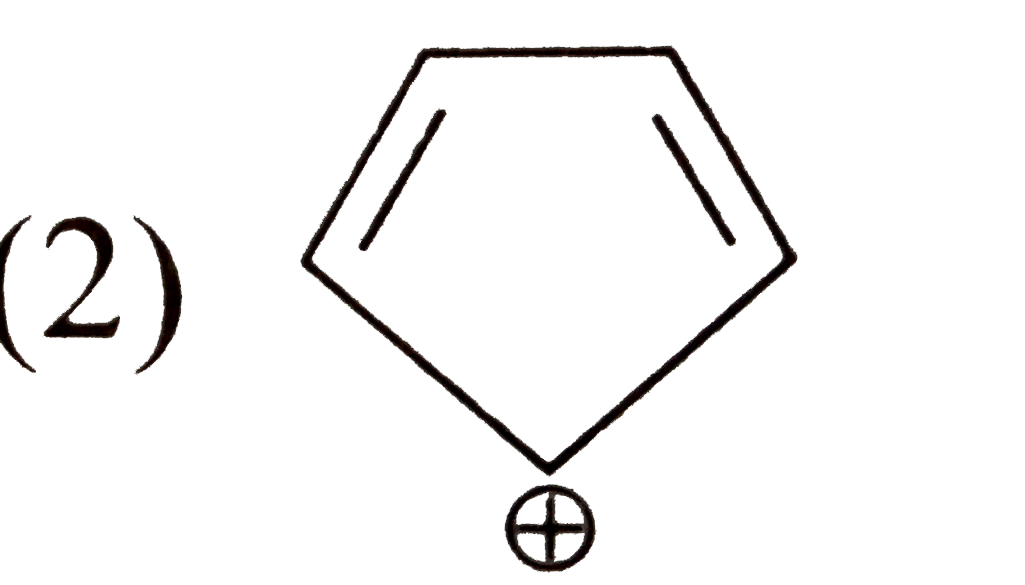 C
C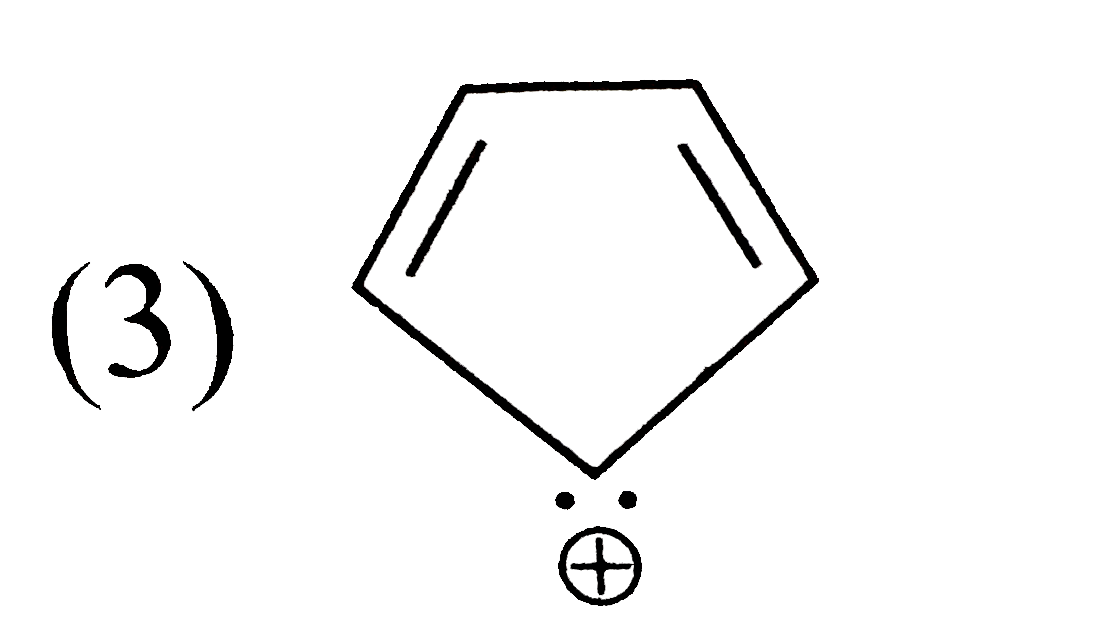 D
D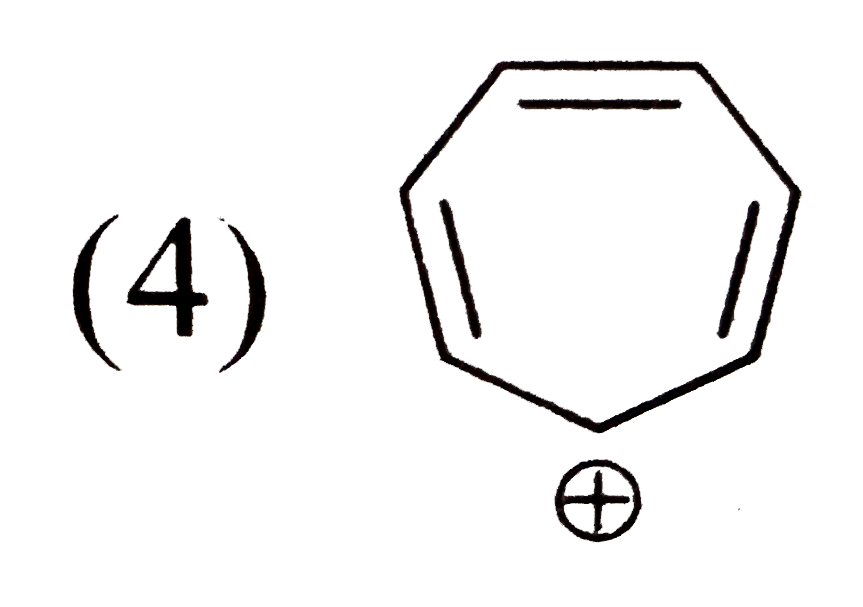
Which of the following is not aromatic?
Acyclopentadienyl cationBcyclopropenyl cationCTropylium cationDCyclopentadienyl anion