The decreasing order of rate of reaction for the following compounds towards SN2Th (bimolecular nucleophilic substitution with tetrahedral intermediate) reaction is
(i) CH3−CH2−O∣∣C−NH2
(ii) CH3−CH2−O∣∣C−Br
(iii) CH3−CH2−O∣∣C−O−O∣∣C−CH2−CH3
(iv) CH3−CH2−O∣∣C−O−C2H5
I>II>III>IV
II>III>I>IV
III>II>IV>I
II>III>IV>I
The correct Answer is:D
Step by step video, text & image solution for The decreasing order of rate of reaction for the following compounds towards S_(N)2Th (bimolecular nucleophilic substitution with tetrahedral intermediate) reaction is (i) CH_(3)-CH_(2)-overset(O)overset(||)C-NH_(2) (ii) CH_(3)-CH_(2)-overset(O)overset(||)C-Br (iii) CH_(3)-CH_(2)-overset(O)overset(||)C-O-overset(O)overset(||)C-CH_(2)-CH_(3) (iv) CH_(3)-CH_(2)-overset(O)overset(||)C-O-C_(2)H_(5) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The molecules CH3−O∣∣C−CH2−O∣∣C−O−C2H5 and CH3−OH∣C−OH−O∣∣C−O−C2H5 are
Ageometrical isomersBTautomersCDiastereomersDMetamersThe IUPAC name of CH3−CH3∣CH−O∣∣C−CH2CH2OH is
A1-Hydroxy-4-methyl pentan-3-oneB2-Methyl-5-hydroxy pentan-3-oneC4-Methyl-3-oxopentan-1-olDHexan-1-ol-3-oneCH3−OH∣CH−O∣∣C−CH2−CH3 will respond to
Aonly Fehling solutionBOnly Tollen's reagentCBoth Tollen's reagent and Fehling solutionDnone of these
The IUPAC name of the compound given below is :
H3C−CH2−O∣∣C−CH2−CH2−O∣∣C−OCH3
View SolutionCH3−O∣∣C−CH3 and CH3−O∣∣C−H is differentiated by:
View SolutionThe IUPAC name for
CH3−O∣∣C−CH2−CH2−O∣∣C−OH is :
View SolutionCH3−O∣∣C−C2H5 & CH3−O∣∣C-
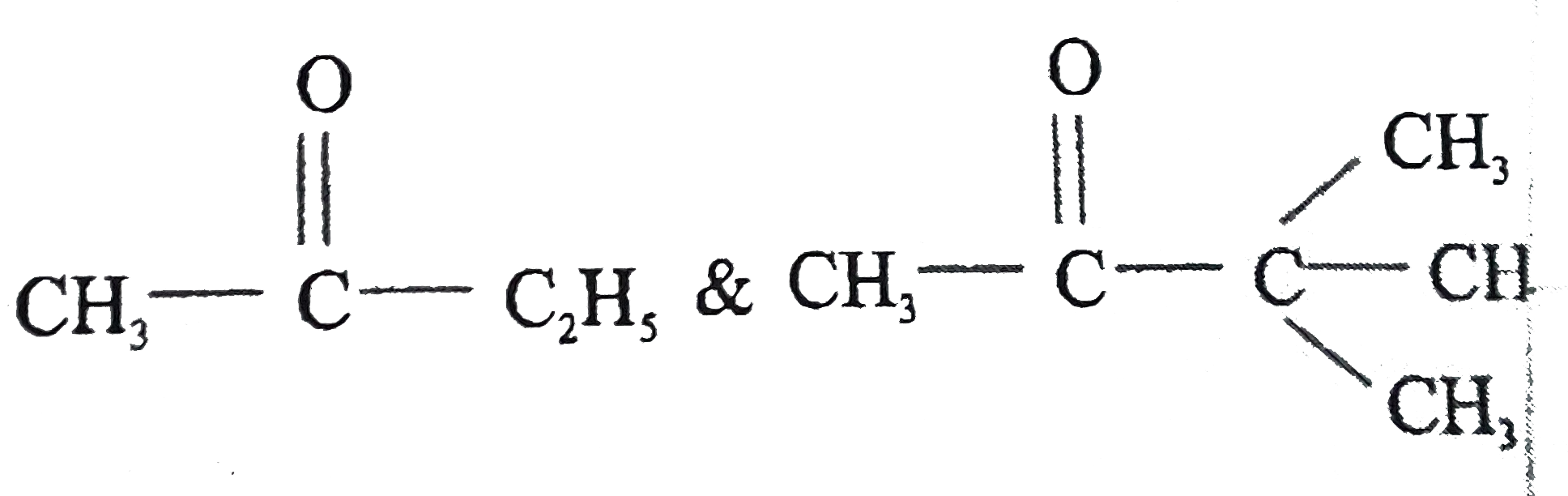
cannot be distinguished by
View SolutionCH3−O∣∣C−H and CH3−O∣∣C−CH3 is differentiated by :
View Solution
