In Maxwell’s velocity distribution curve area under the graph
Increases when temperature is increased
Decreases when temperature is increased
remains same at all temperature
depends on the pressure of the gas
Step by step video & image solution for In Maxwell’s velocity distribution curve area under the graph by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Read the following paragraph and answer the questions given below:
According to Maxwell distribution, the area under the curve is equal to the total number of molecules in collision. On increasing temperature, fraction of molecules having speed equal to ump (most probable speed) decreases. The speed distribution also depends on the mass of the molecules along with the temperature.
In general, the distribution depends upon the value of MT (where M is molar mass and T is temperature in kelvin).
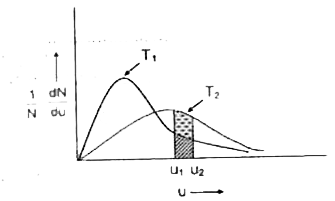
Choose the correct option:
On increasing temperature, which of the following statement is incorrect?AArea under the curve at different temperature are different.BFraction of the molecules having speed equal to ump decreases.CMore molecules possess speeds near to the most probable speed.DThe distribution of speed depends only on the value of M (M = molar mass, T in kelvin).In maxwell's speed distribution curve, for N2 gas, the average of |relative velocity| between two molecules at 300 k will be : -
A300 m/secB610 m/secC920 m/secDzeroThe gas molecules randomly move in all directions and collide with each other and with the wall of the container. If is difficult to determine the speed of an individual molecule but it had become passible to work out the distribution of molecules among different molecular speeds. This is known as Maxwell Boltzmann distribution
consider the following graph about Maxwell's distribution of speed at two different temperature T1 and T2
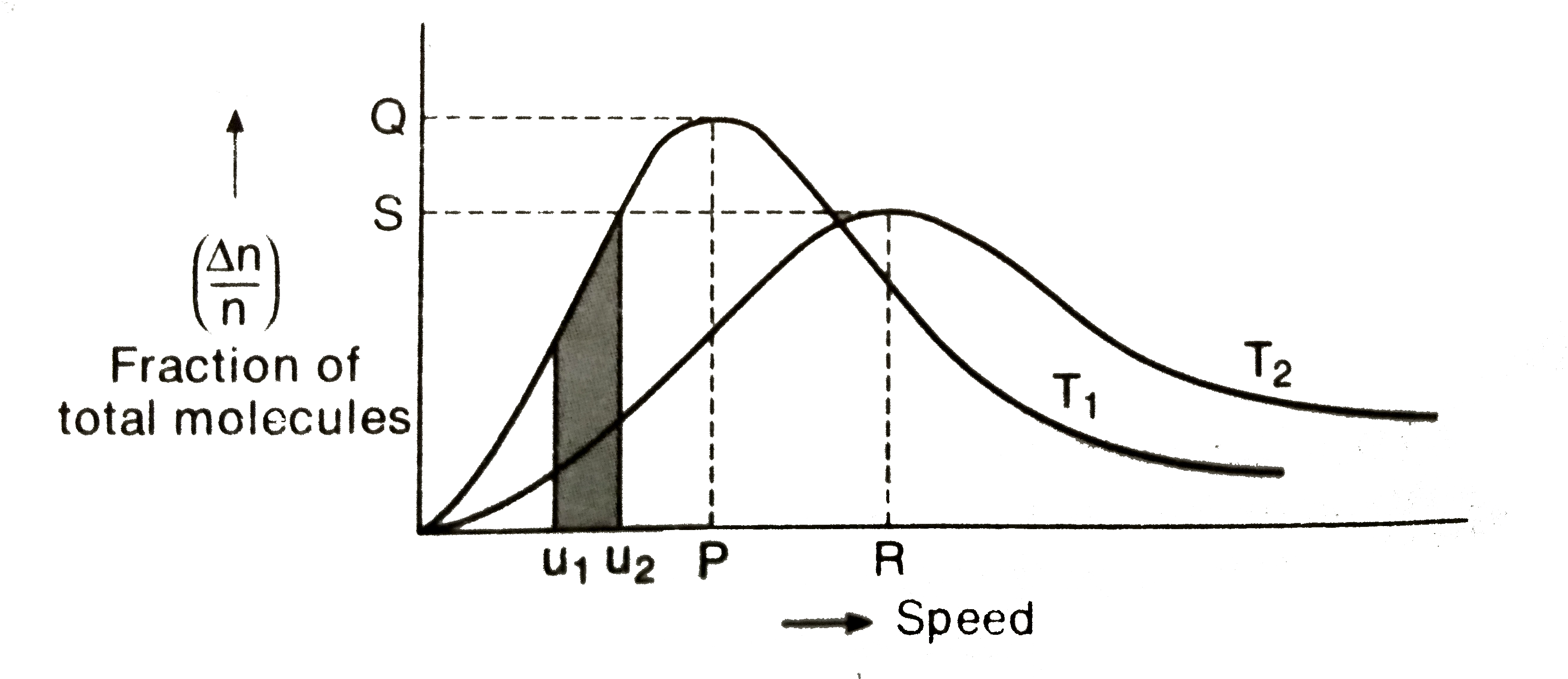
Total area under the curve at T1 is :Aequal to that under curve at T2Bless than that under curve at T2Cgreater than that under curve at T2Dcan be greater or less than that under curve at T2, depending on the nature of the gas
Area under Curve
View SolutionArea under curves
View SolutionThe gas molecules randomly move in all directions and collide with each other and with the wall of the container. If is difficult to determine the speed of an individual molecule but it had become passible to work out the distribution of molecules among different molecular speeds. This is known as Maxwell Boltzmann distribution
consider the following graph about Maxwell's distribution of speed at two different temperature T1 and T2
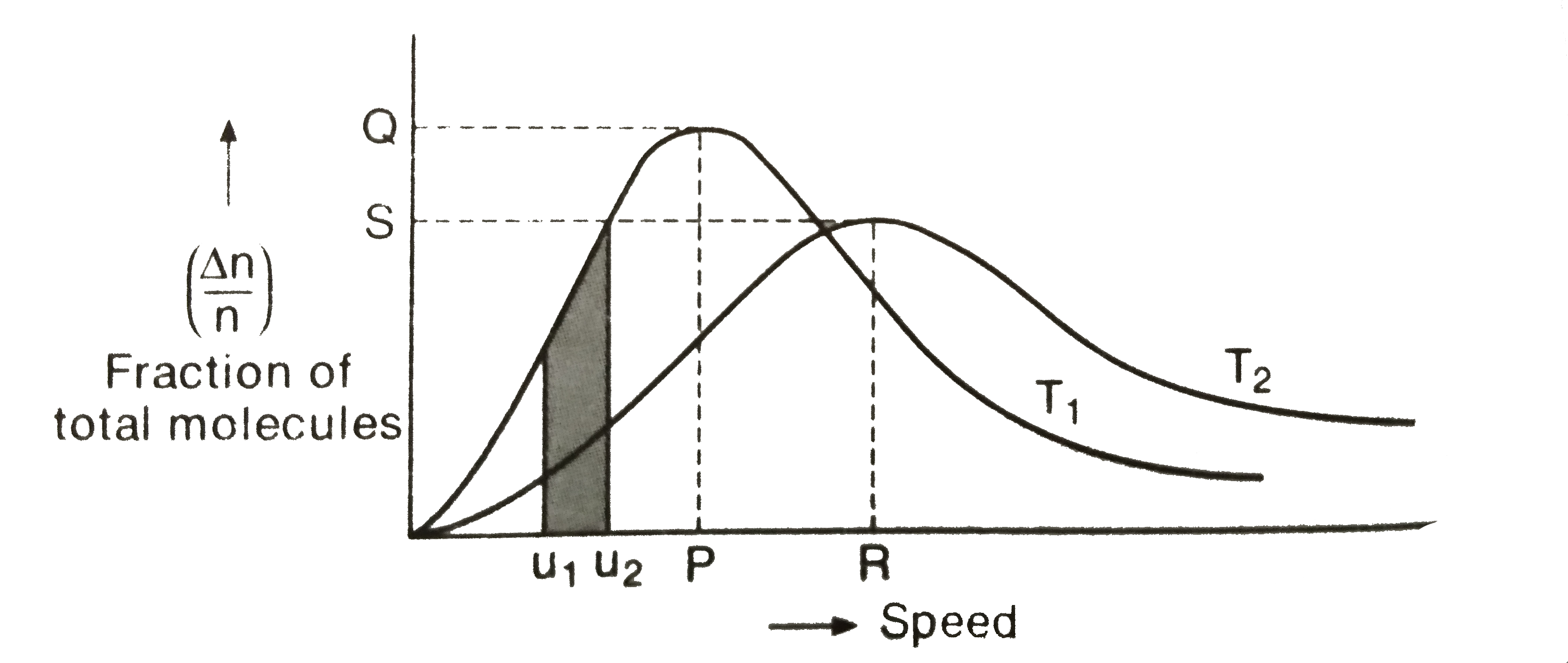
The shaded area represents :
View SolutionThe gas molecules randomly move in all directions and collide with each other and with the wall of the container. If is difficult to determine the speed of an individual molecule but it had become passible to work out the distribution of molecules among different molecular speeds. This is known as Maxwell Boltzmann distribution
consider the following graph about Maxwell's distribution of speed at two different temperature T1 and T2
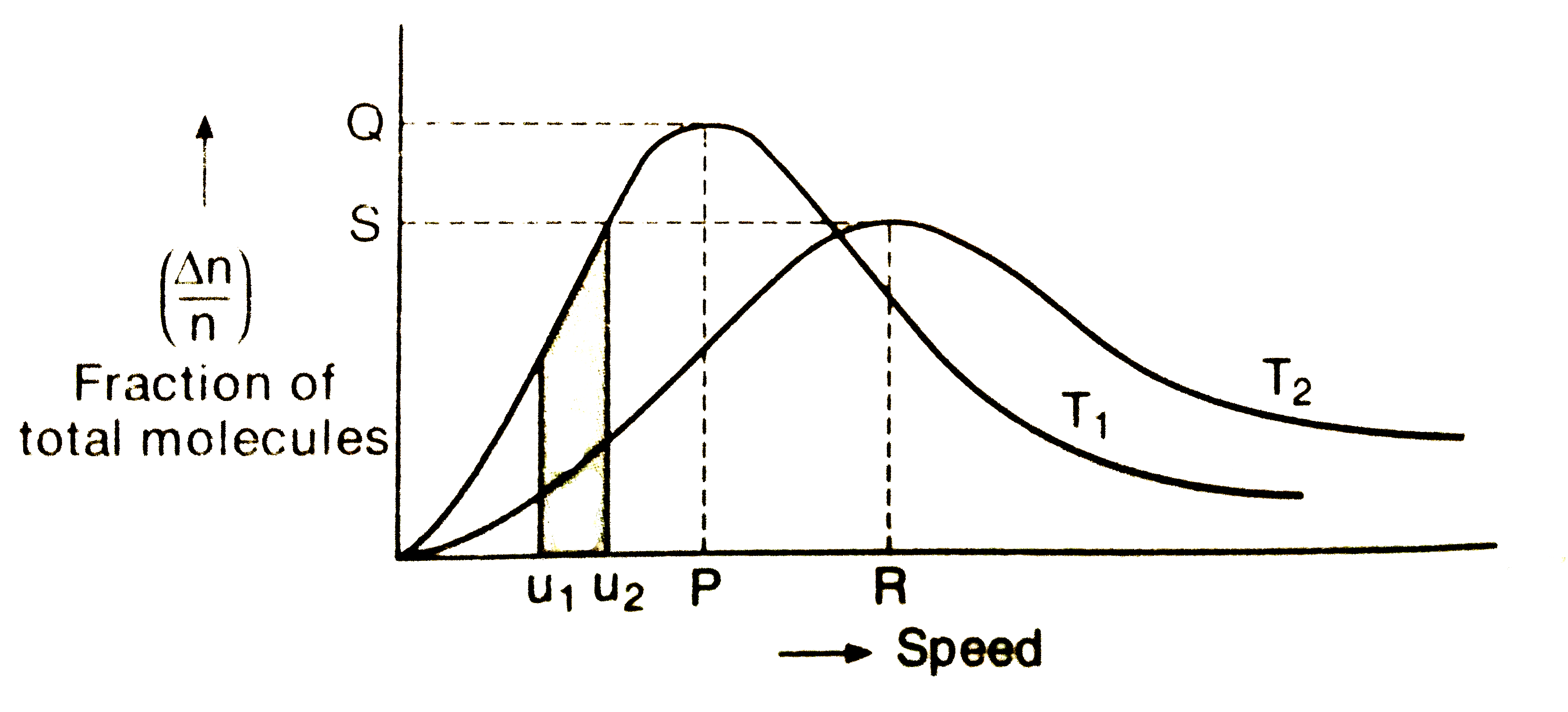
Select the correct statement (s):
View SolutionThe gas molecules randomly move in an directions and collide with each other and with the wall of the container. It is dificult to detemine the speed of an individual molecule but it has become possible to Work out the distribution of molecules among different molecular speeds. This is known as Maxwell
Boltzmann distribution.
Consider the following graph about Maxwell's distribution of speeds at two different temperatures T1andT2 and answer the following questions:
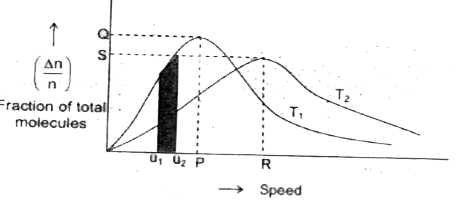
Total area under the curve at T1 is
View Solution
