K1 and K2 are the rate constants of forward and backward reactions. The equilibrium constant K of the reaction is -
K1×K2
K1−K2
K1K2)
K1+K2K1−K2
The correct Answer is:C
Step by step video, text & image solution for K_(1) and K_(2) are the rate constants of forward and backward reactions. The equilibrium constant K of the reaction is - by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
In terms of rate constants for forward and backward reactions (kfandkb) , equilibrium constant of a reaction is equal to …………..
View SolutionIn a reaction A+B⇔C+D the rate constant of forward reaction & backward reaction is K1 and K12 then the equilibrium constant (K) for reaction is expressed as:
View Solution
Knowledge Check
k1andk2 are the velocity constants of forward and backward reactions. The equilibrium constant K of the reaction is
Ak1×k2Bk1−k2Ck1/k2Dk1+k2k1−k2Kf and Kb are the velocity constants of forward and backward reactions. The equilibrium constant Keq of the reversible reaction will be
AKb/KfBKf×KbCKf/KbDKf−KbIn a reaction A+B⇔C+D the rate constant of forward reaction & backward reaction is K1 and K2 then the equilibrium constant (K) for reaction is expressed as:
AKc=K2K1BKc=K1K2CKc=K1×K2DKc=K1+K2
The equilibrium constant (K) of a reaction may be written as
View SolutionThe equilibrium constant (K) of a reaction may be written as
View SolutionThe energy change accompanying the equilibrium reaction A⇔B is −33.0kJmol−1.
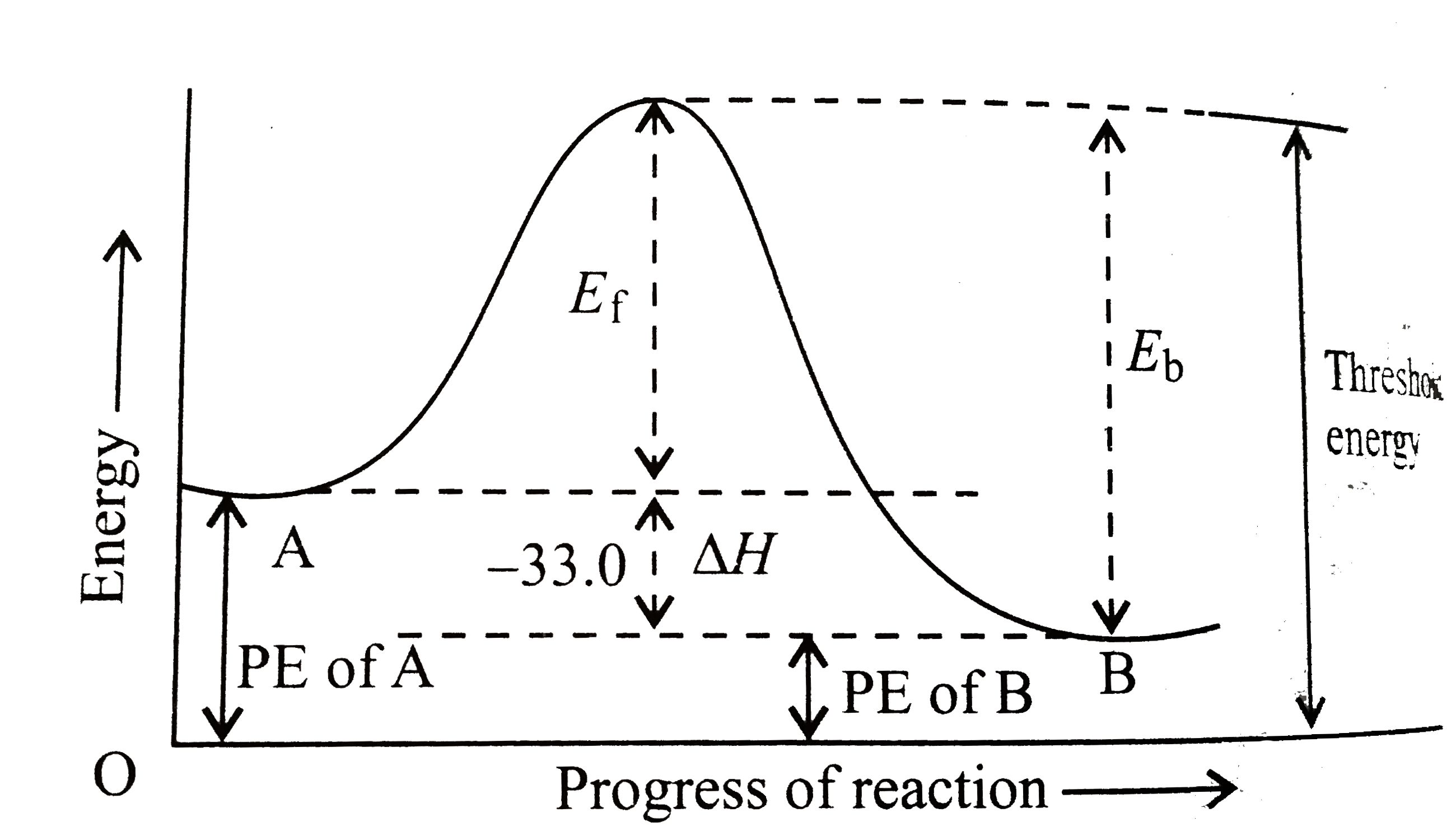
Assuming that pre-exponential factor is same for forward and backward reaction answer the following:
The equilibrium constant k for the reaction at 300K
View SolutionIn a chemical equilibrium, the rate constant for the backward reaction is 7.5×10−4 and the equilibrium constant is 1.5 the rate constant for the forward reaction is :
View SolutionIn a chemical equilibrium, the rate constant for the backward reaction is 7.5×10−4 and the equilibrium constant is 1.5 the rate constant for the forward reaction is:
View Solution
