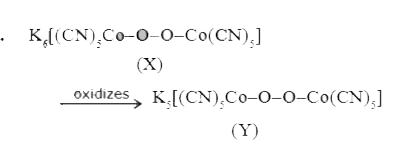
In both the complexes Co cation have t62ge0g configuration.
The B. E. of (O–O) in X & Y is
B. E. of (O – O) in y lt B. E of (O – O) in X
B. E. of (O – O) in X lt B. E. of (O – O) in Y
B. E. of (O – O) in X = B. E. of (O – O) in Y
can’t be compared
The correct Answer is:B
Step by step video, text & image solution for In both the complexes Co cation have t_(2g)^6 e_g^0 configuration. The B. E. of (O–O) in X & Y is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
[(H2O)4Co(O2)2Co(H2O)4](SO4)2[X]reagent−−−−−−→[(H2O)4Co(O2)2Co(H2O)4](SO4) (I) (II)
In both the complex Co has t62geg0 configuration. Which option is incorrect ?
View SolutionWhich of the following statements is/are correct? (i) In octahedral complexes, t_(2g) orbitals posses low energy as compared to e_g orbitals (ii) In tetrahedral complexes , t_2 orbitals posses high energy as compared to e orbitals (iii) In octahedral complexes , e_g orbitals possess low energy as compared to t_(2g) orbitals
View SolutionWhat are t2g and eg orbitals ?
View Solutiont_(2g)^6 e_g^2 Magnetic moment of configuration (mu) will be honoured :
View Solutionसभी धातु आयन t62ge0g विन्यास रखते हैं। निम्न में से कौनसा संकुल अनुचम्बकीय होगा?
View Solutionजब संकुल K6[(CN)5Co−O−O−Co(CN)5] ब्रोमीन द्वारा K5[(CN)5Co−O−O−Co(CN)5] में ऑक्सीकृत होता है तो इस परिवर्तन के सन्दर्भ में निम्न में से कौनसा कथन सही है -
(दोनों संकुलों में Co का विन्यास t 62g,e0,0g है )
View SolutionWhat are t2g and eg orbitals ?
View Solution
