If the number of electrons in p-orbital are two, then which one of the following is in accordance with Hund’s rule -
A
p2xp0yp0z
B
p0xp2yp0z
C
p0xp0yp2z
D
p1xp1yp0z
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for If the number of electrons in p-orbital are two, then which one of the following is in accordance with Hund’s rule - by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Which of the following is a violation of the Hund's rule?
A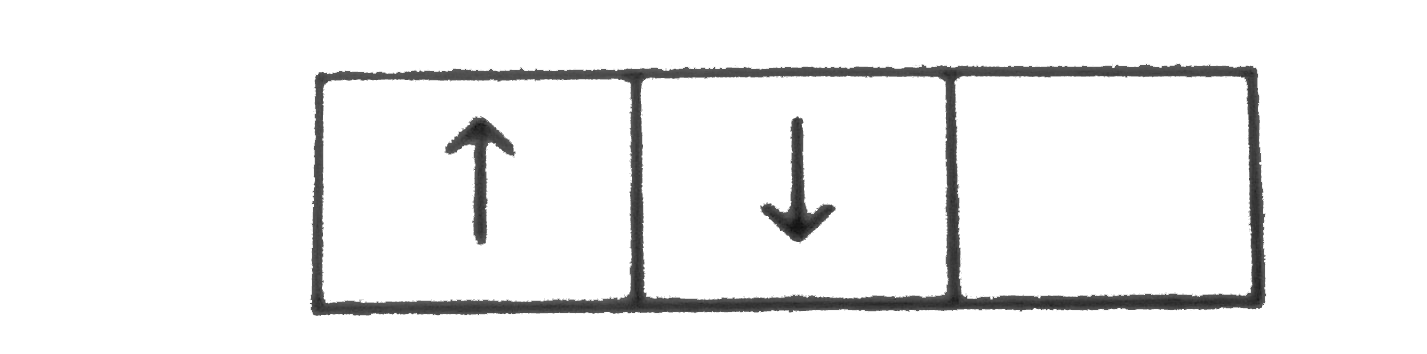 B
B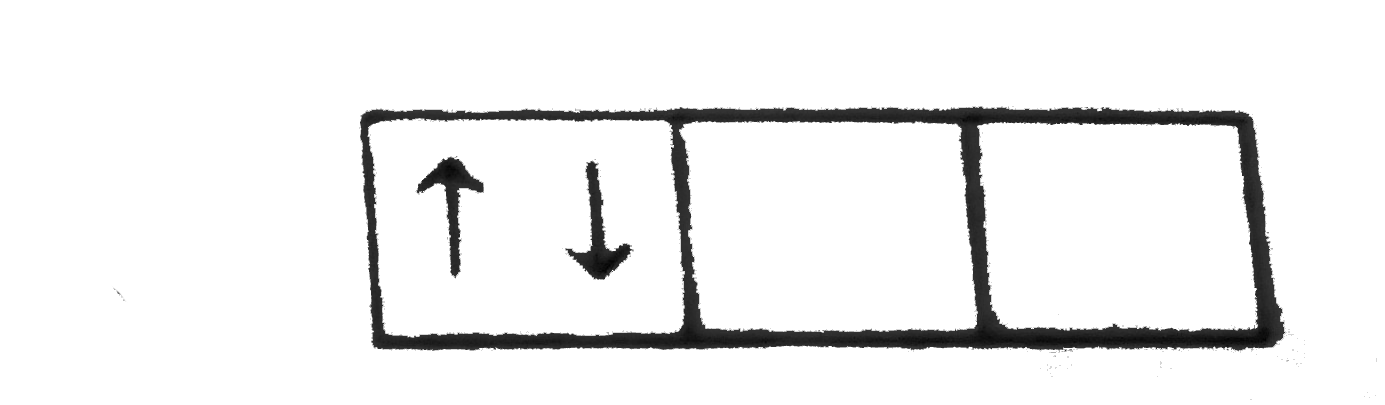 C
C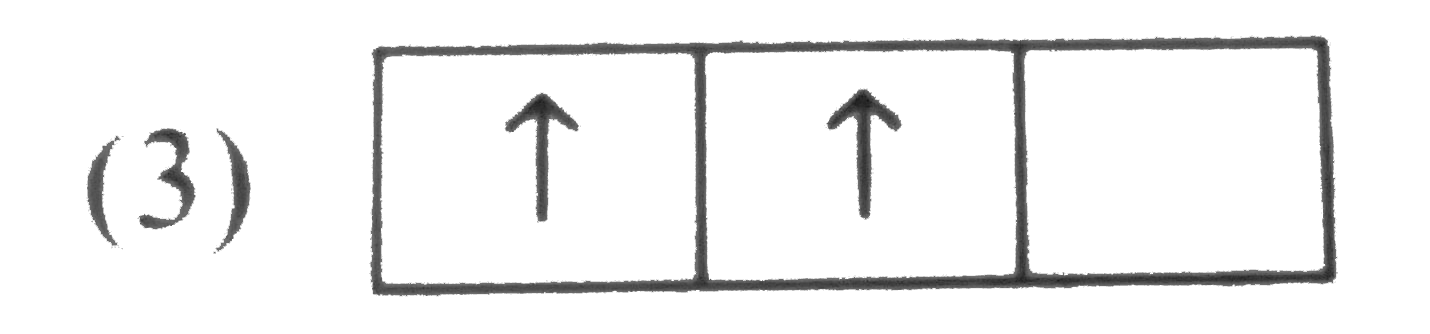 DBoth (1) and (2)
DBoth (1) and (2)Which of the following is violating hund's rule :-
A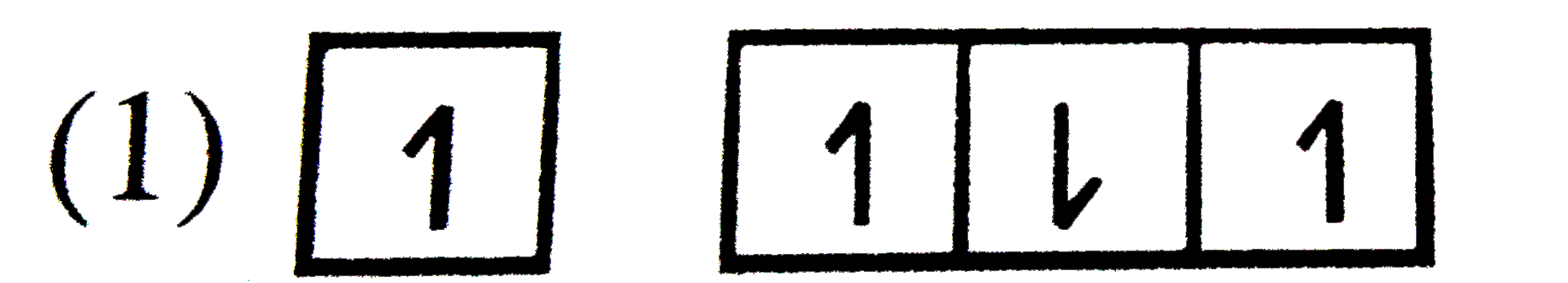 B
B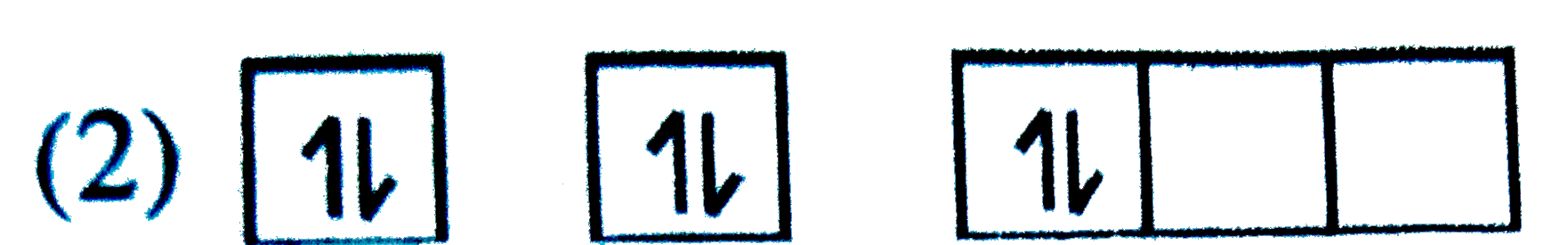 C
C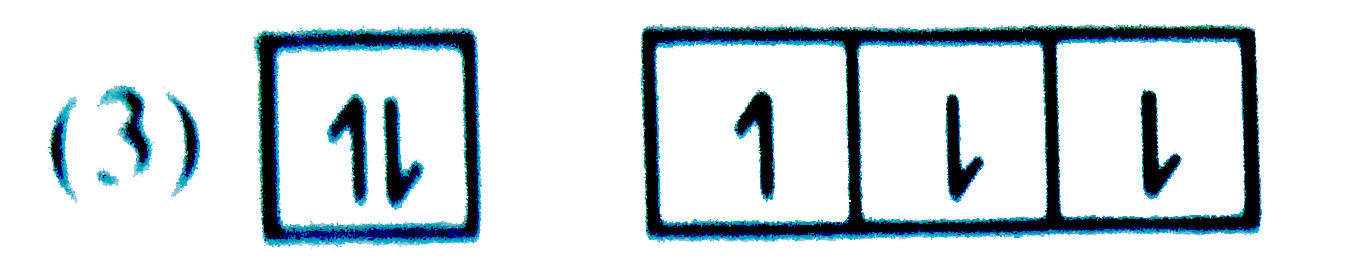 Dall
DallIf Hund Rule is violate, then which of the following species is diamagnetic -
ASe+BCu+2CFe+2DCr+1
If Hund's rule is not followed then which of the following ion is paramagnetic,
View SolutionThe orbital diagram in which both the Pauli's exlusion principle and Hund's rule violated is
View SolutionHund's rule is violated in which of the following electronic configurations?
View SolutionHund's rule is violated in which of the following electronic configurations?
View SolutionHund's rule is violated in which of the following electronic configurations?
View Solution
