Select the correct curve(s) :
If v = velocity of electron in Bohr’s orbit.
r = Radius of electron in Bohr’s orbit.
P.E. = Potential energy of electron in Bohr’s orbit.
K.E. = Kinetic energy of electron in Bohr’s orbit.
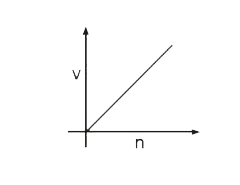
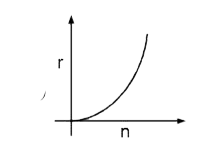
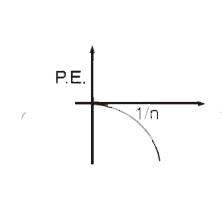
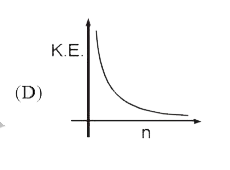
The correct Answer is:B, C, D
Similar Questions
Select the correct curve (s) :
If=V Velocituy of electron in Bohr's orbit
r= Radius of electron in Bohar's orbit
P.E= Potential energy of electron in Bohr's orbit
K.E.= Kinetic energy of electron in Bohr's orbit
View SolutionDraw correct curve for V vs 1/r :
If=V Velocituy of electron in Bohr's orbit
r= Radius of electron in Bohar's orbit
P.E= Potential energy of electron in Bohr's orbit
K.E.= Kinetic energy of electron in Bohr's orbit
View Solution
Knowledge Check
Select the correct cruve (s)
If V=velocity of electron in Bhor's orbit ltbr> R= Radius of electron im Bhor 's orbit
P.E= Potential energy of electron in Bhor 's orbit
K.E.=kinetic energy of electron in Bhor 's orbit.A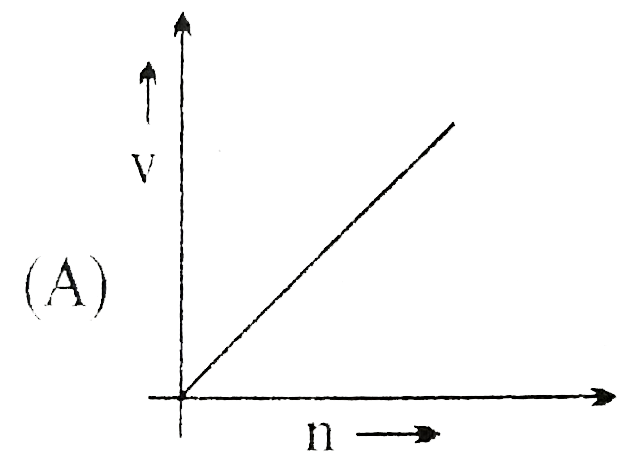 B
B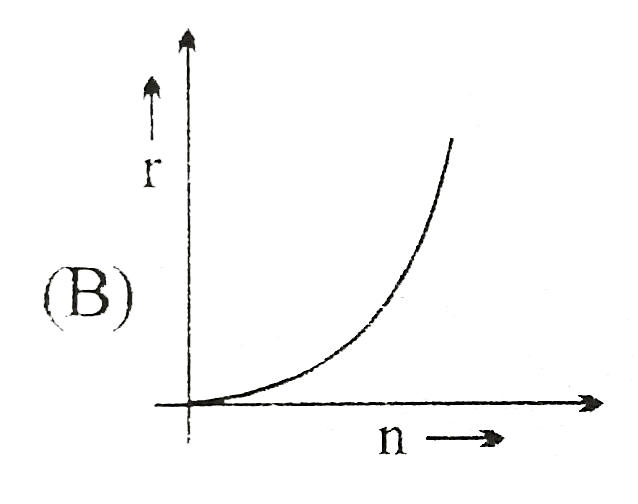 C
C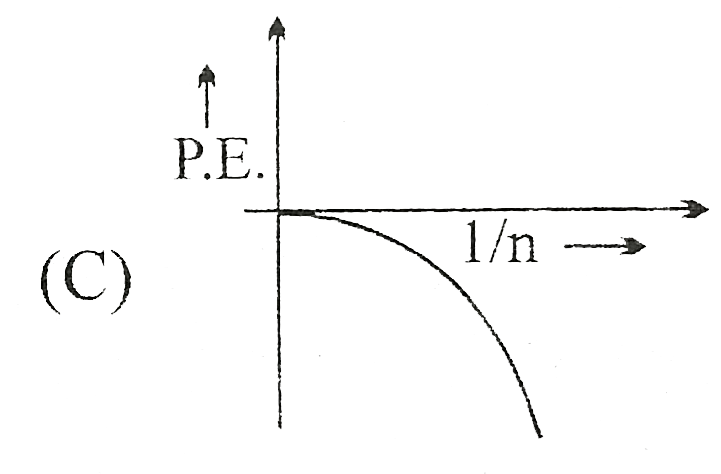 D
D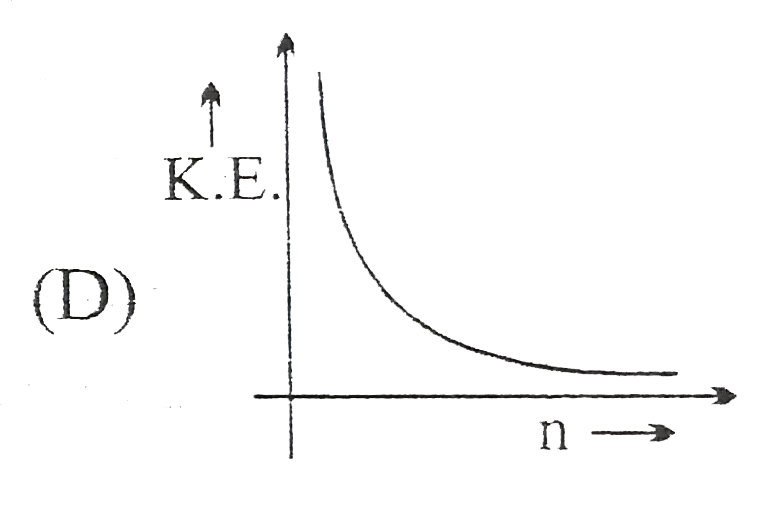
Which is the correct relation between energies of one electron species?
E1 =Energy of electron in the first Bohr orbit,
E2 =Energy of electron in the second Bohr orbit,
E3 =Energy of electron in the third Bohr orbit,
E4 =Energy of electron in the fourth Bohr orbit.AE1 of H=1/2E2ofHe+=1/3E3 of Li2+=1/4E4 of Be3+BE1(H)=E2(He+)=E3(Li2+)=E4(Be3+)CE1(H)=2E2(He+)=3E3(Li2+)=4E4(Be3+)DNone of the above relations are correctIf the PE of an electron in the first Bohr orbit of H is zero , the total energy of the electron in second Bohr orbit is
A23.8eVB−23.8eVC−3.4eVD3.4eV
Bohr's Model(energy of electron in an orbit)
View SolutionEnergy OF electron in nth bohr orbit,ionisation energy
View SolutionCalculate the radius of the third Bohr orbit of hydrogen atom and the energy of electron in that orbit.
View SolutionIf the PE of an electron in the first Bohr orbit of H is zero , the total energy of the electron in second Bohr orbit is
View SolutionIf the energy of an electron in 3rd Bohr orbit is −E, what is the energy of the electron in (i) 1st Bohr orbit (ii) 2nd Bohr orbit ?
View Solution

