Solution of chemical compound X reacts with AgNO3 solution to form a white ppt. Y which dissolves in NH4OH to give a complex Z. When Z is treated with dil. HNO3, Y reappears. The chemical compound X can be-
NaCl
CH3Cl
NaBr
NaI
The correct Answer is:A
Step by step video, text & image solution for Solution of chemical compound X reacts with AgNO_(3) solution to form a white ppt. Y which dissolves in NH_(4)OH to give a complex Z. When Z is treated with dil. HNO_(3), Y reappears. The chemical compound X can be- by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Aqueous solution of metallic nitrate X reacts with NH4OH to form Y which dissolves in excess NH4OH. The resulting complex is reduced by acetaldehyde to deposit the metal. X and Y, respectively, are
View SolutionA: The complex (CONH,Cl gives white ppt with AgNO3 solution
R: Chlorine always gives white ppt with AgNO3 solution
View Solution
Knowledge Check
The solution of a chemical compound X reacts with AgNO3 solution to form a white precipitate of y which dissolves or NH4OH to give Z When Z is treated with dil HNO3 ,Y resppears .The chemical compound X can be
ANaCIBCH3CICNaBrDNaTAqueous solution of a salt when treated with AgNO3 solution gives a white precipitate , white dissolves in NH4OH. Radical present in the salt is
ACl−BS2−CI−DAll of themWhen calomel react with NH4OH solution the compound formed is
ANH2−Hg−ClBHg2Cl2NH3CHg(CH3)2Cl2DHgCl2NH3
Which of the following compounds, when heated with HNO3 (conc), cooled and then treated with AgNO3 a white pot, is formed. The compound can be
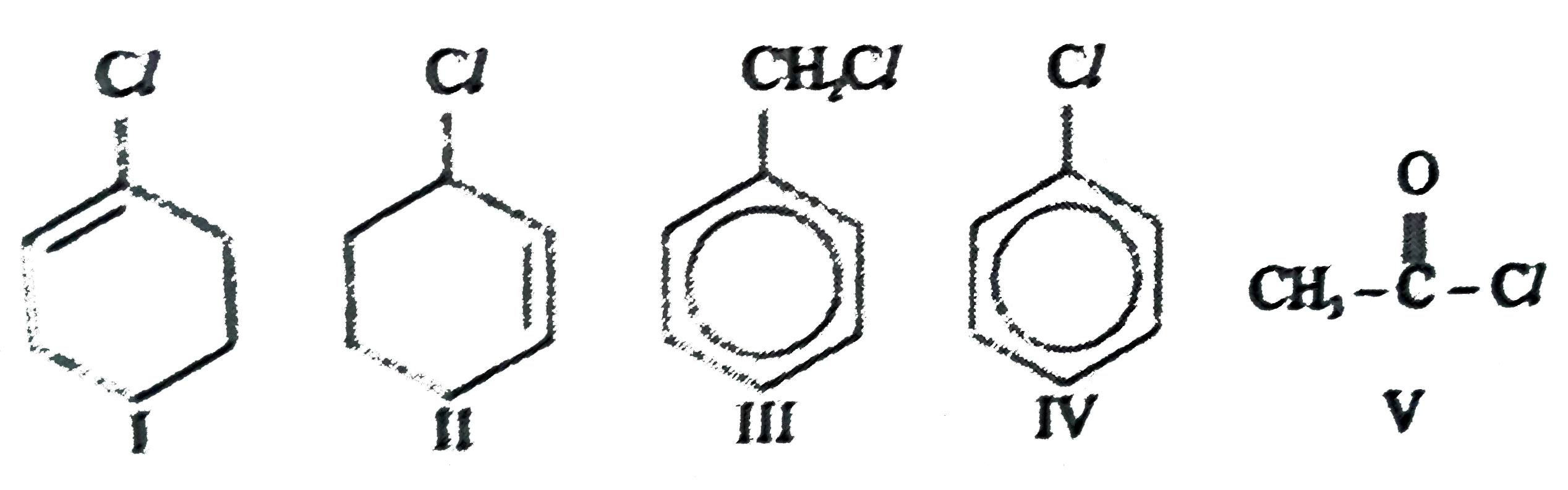
View SolutionA compound X, of boron reacts with NH3 on heating to give another compound Y which is called inorganic benzene. The compound X can be prepared by treating and Y are represented by the formula.
View SolutionA metal M reacts with sodium hydroxide to give a white precipitate X which is soluble in excess of NaOH to give Y. Compound X is soluble in HCI to form a compound Z. Identify M.X,Y and Z
M , X , Y, Z
View SolutionA solution of a compound X in dilute HCl on treatment with a solution of BaCl2 gives a white precipitate of compound Y which is insoluble in conc. HNO3 and and conc. HCl. Compound X imparts golden yellow colour to the flame.
X(Solution in dilute HCl)(immparts golden yellow colour)+BaCl2→YWhiteConc. HNO3−−−−−−−→Conc. HClInsoluble
What are compounds X and Y?
View SolutionA metal complex having composition [Cr(NH3)4Br2] was isolated in two forms (X) and (Y). Form (X) reacts with AgNO3 to gives a plate yellow precipitate which is partially soluble in excess of NH4OH whereas (Y) gives a greenish yellow precipitate which is insoluble in NH4OH.
The formula of X and Y are
View Solution
