The following graph is obtained for physisorption of a gas as a function of pressure at different temperatures.
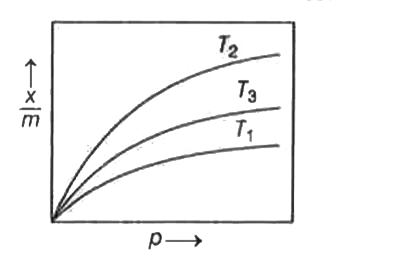
The correct order of temperatues is Solution in Telugu
T3<T2<T1
T2<T3<T1
T2<T1<T3
T1<T3<T2
The correct Answer is:B
The degree of absorption increases with the decrease in temperature. The correct order of temperature is T2<T3<T1.
Similar Questions
Following graph is obtained for four different reactions On the basis of above graph, correct increasing order of reaction is
View SolutionThe volume - temperature graph of a given mass of an ideal gas at respective constant pressure is shown below. What is the CORRECT order of pressure ?
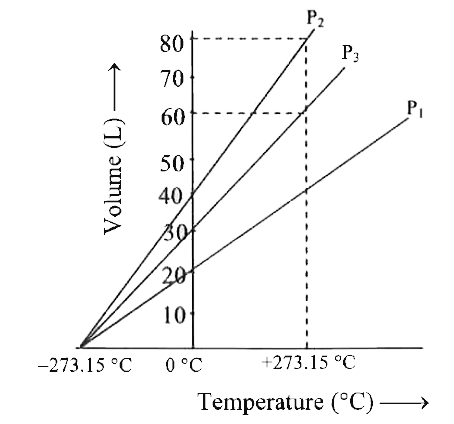
View SolutionFrom the graph the order of pressure of a gas is
View SolutionThe volume-temperature graphs of a given mass of an ideal gas at constant pressure are shown below. What is the correct order of pressure ?
View SolutionCompressibility factor (Z) is plotted against pressure at different temperature for same gas

Which of the following is the correct order of temperature shown in the above plot ?
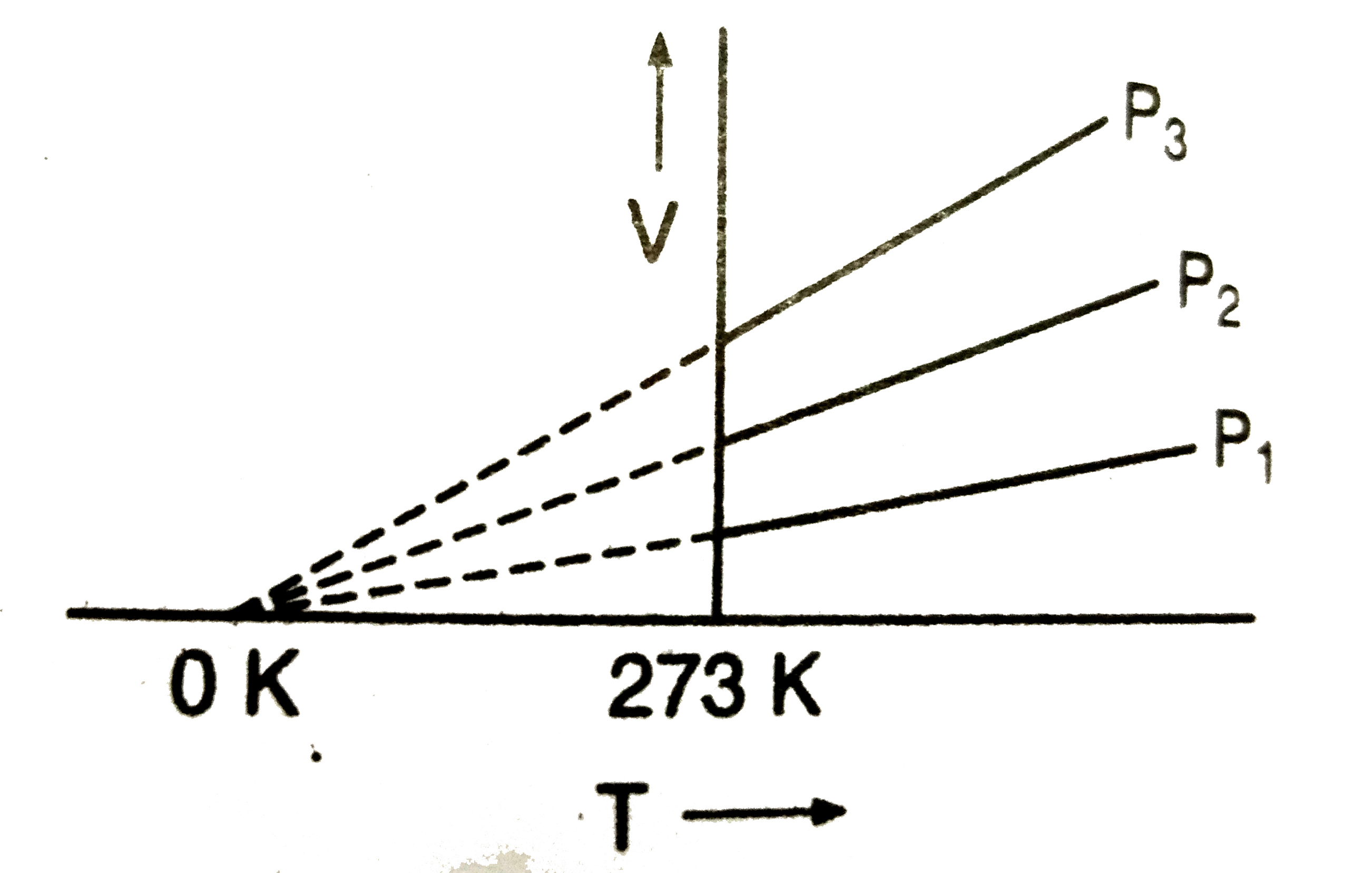
View SolutionThe volume-temperature graph for a given mass of an ideal gas at constant pressure is shown below. What is the correct order of pressure?
View SolutionThe compressibility coefficient (z) is plotted with respect to pressure at different temperatures for the same gas. Which of the following is the correct order of temperature shown in the above graph?
View SolutionWhich of the following is the correct graph of the volume of a fixed volume of ideal gas as a function of temperature (at constant pressure)?
View Solution

