Which of the following metal ions has a calculated magnetic moment value of √24 BM ?Solution in Telugu
Mn2+
Fe2+
Fe3+
Co2+
The correct Answer is:b
We know that, magnetic moment (μ) is related to number ofunparied electron n, as μeff=√n(n+2)BM
μeff=√24BM
This corresponcs, n=4 as μeff=√4(4+1)=√24
=2.√2.Sqrt(3)
=2×1.414×1.732
= 4.89 Bm
(Trick) = Number of unpaired e− are equal to value of variable number of BM)
Out of given option, Fe2+ has 4 unparied electrons as
Fe2+=[Ar]=
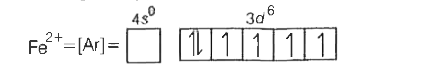
Topper's Solved these Questions
Similar Questions
When one mole of A and one mole of B were heated in a one litre flask at T(K), 0.5 moles of C were formed at equilibrium
A+B⇔C+D
The equilibrium constant KC is
View SolutionWhich of the following conditions are correct for real solutions showing negative deviation from Raoult's law?
View SolutionWhich of the following ion has magnetic moment is 3.87 BM :-
View SolutionWhich of the following metal ions has a calculated magnetic moment value of √24 BM ?
View Solutionप्रथम संक्रमण श्रेणी के एक धातु आयन का चुंबकीय आघूर्ण ( गणना द्वारा प्राप्त ) 3.87 BM है। आयन में उपस्थित अयुग्मित इलेक्ट्रॉनों की संख्या है -
View SolutionThe species with spin only magnetic moment of sqrt(24) BM is:
View SolutionThe species with spin only magnetic moment of sqrt(24) BM is:
View SolutionWhich of the following ions has a magnetic moment of 5.93 BM ?
View SolutionWhich of the following has a magnetic moment of 1.75 BM
View SolutionWhich ion has 5.93 BM magnetic moment?
View SolutionMn^(x+) has a magnetic moment of 4.9 BM. Calculate the value of x in the given ion.
View SolutionAn ion Mna+ has the magnetic moment equals to 4.9 BM what is the value of 'a'?
View SolutionThe species with spin only magnetic moment of sqrt(24) BM is:
View SolutionWhich of the following ions has a magnetic moment of 5.93 BM? (At. No. V=23, Cr=24, Mn=25, Fe=26 )
View SolutionA compound in which a metal ion M^(x+) (Z=25) has a spin only magnetic moment of sqrt(24)BM . The number of unpaired electrons in the compound and the oxidation state of the metal ion are respectively
View Solution
