The vapour pressure of a non- ideal two component solution is given below Identify the correct T-X curve for the same mixture,

Identify the correct T-X curve for the same mixutre.




The correct Answer is:A
Answer(1) Vapour pressure α 1/Boiling point
option (1) shows that relation.
Topper's Solved these Questions
Similar Questions
A force F is applied in a square plate of length L . If the percentage error in the determination of L is 3% and in F in 4% then permissible error in the calculation of pressure is
View SolutionIn the Millikan's oil drop method, which of the following force does not act on the oil drop?
View SolutionAn equation of a line whose segment between the coordinates axes is divided by the point (12,13) in the ratio 2:3 is
View SolutionGiven P−x curve for a non-ideal liquid mixture (Fig.). Identify the correct T−x curve for the same mixture.
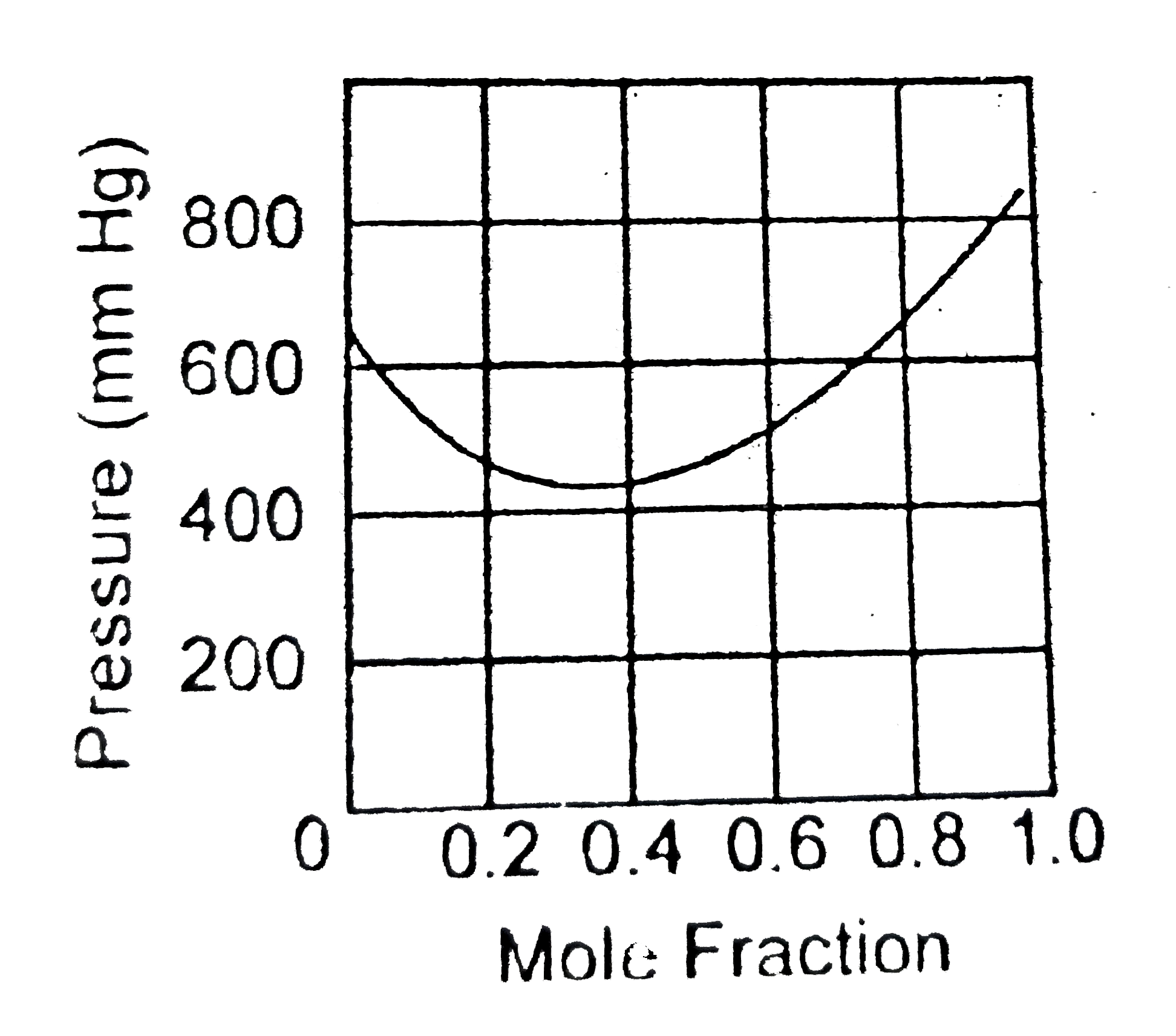
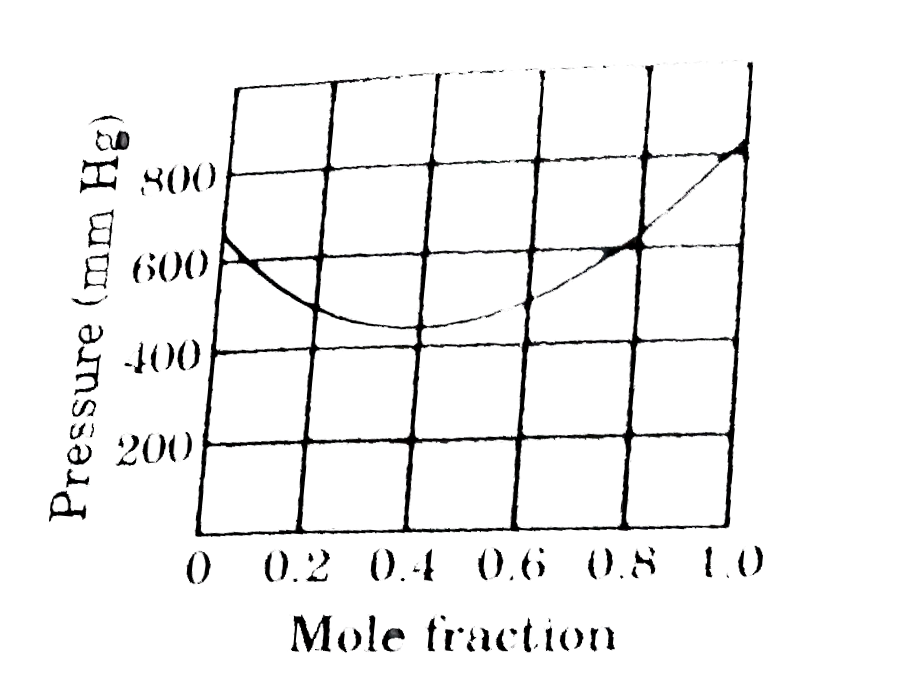
View SolutionGiven P-X curve for a non-ideal liquid mixture (fig). Identify the correct T-X curve for the same mixture.
View SolutionIdentify the cell organelle 'A' :-
ltimg src="https://d10lpgp6xz60nq.cloudfront.net/physics_images/ALN_BIO_C10_E08_018_Q01.png" width="80%"gt
View SolutionWhich of the following are correct chain isomers of butane ? (i)
View SolutionDetermine the point of symmetry of a regular hexagon.
View SolutionMatch the following Column A to Column B

View SolutionMatch the following Column A to Column B
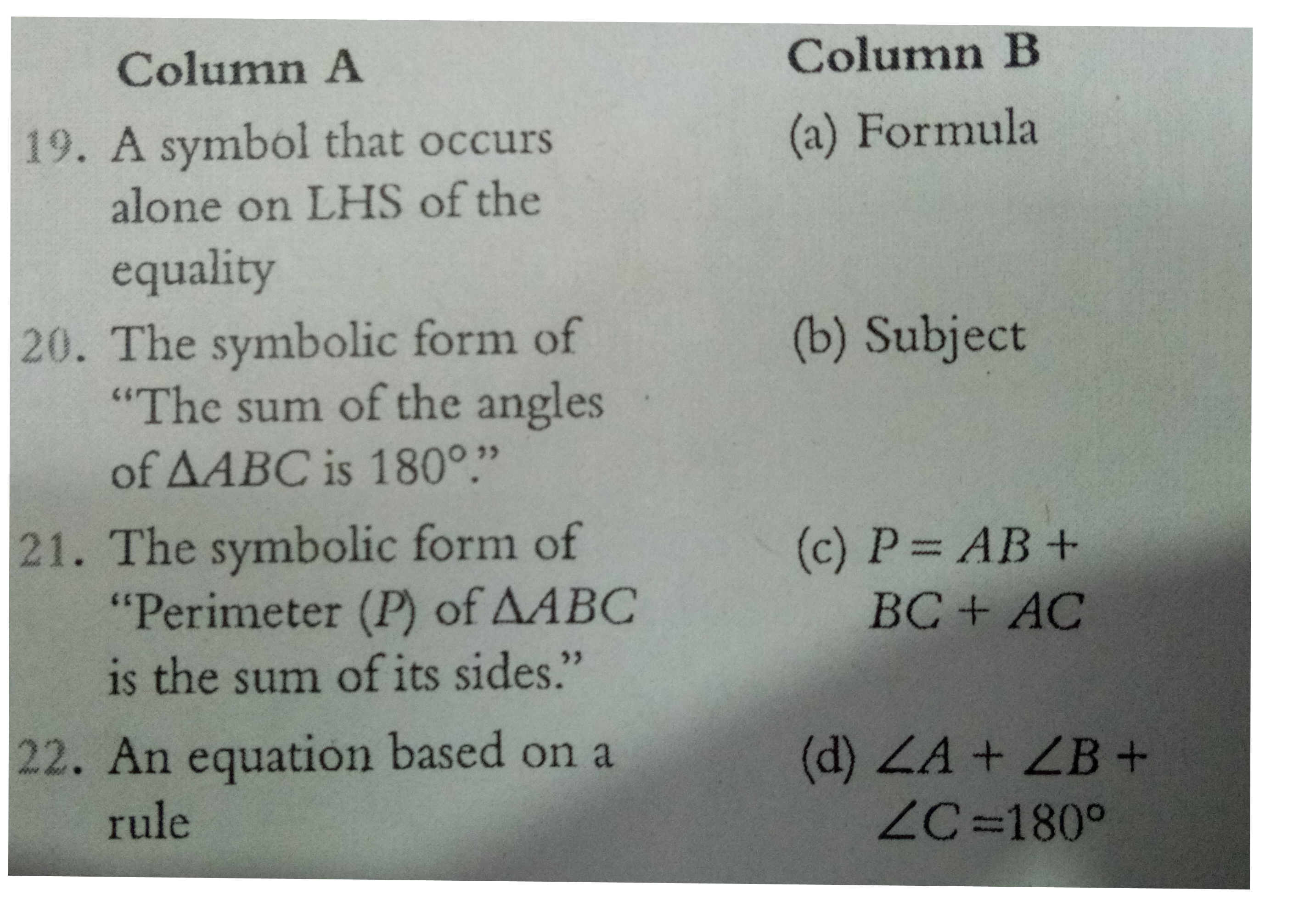
View SolutionMatch the following Column A to Column B

View SolutionFind the area of figure given below.
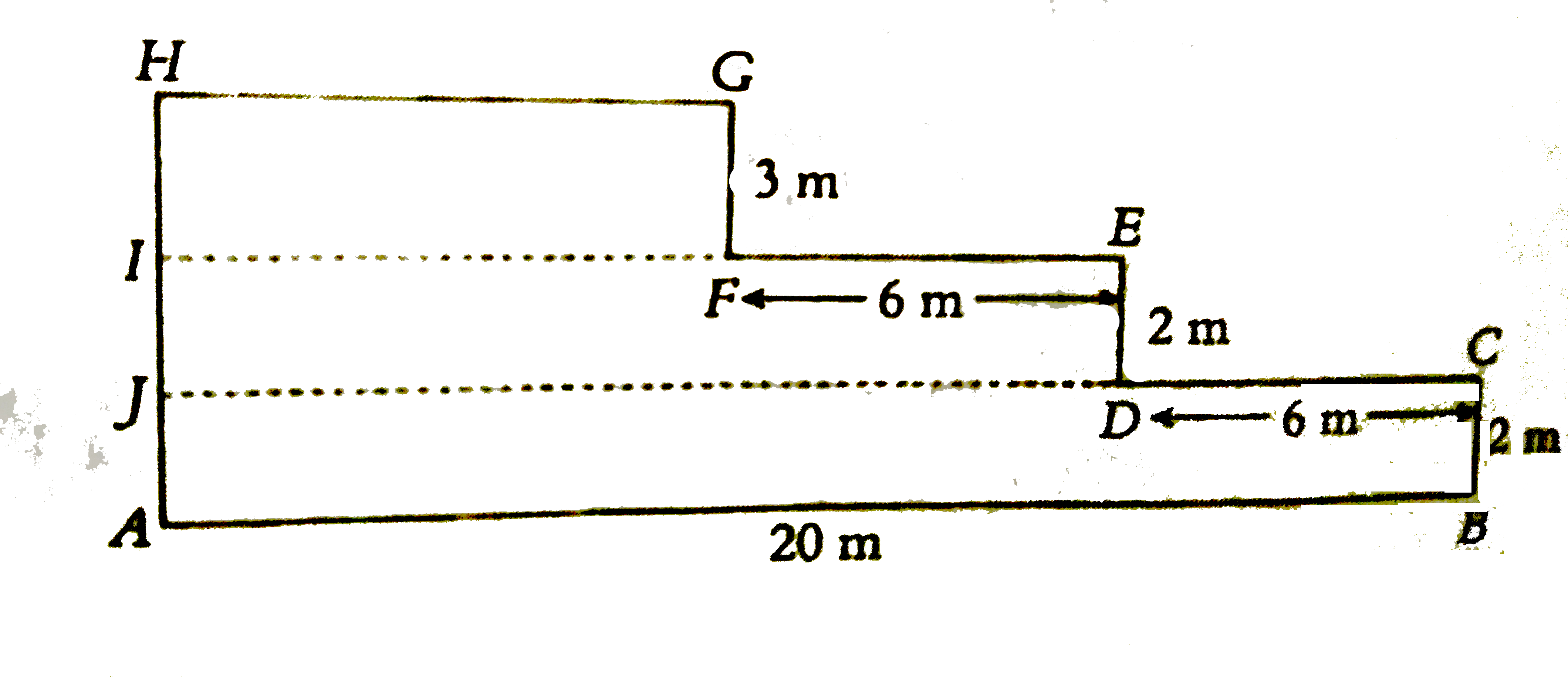
View SolutionFind the perimeter of the figure given below.
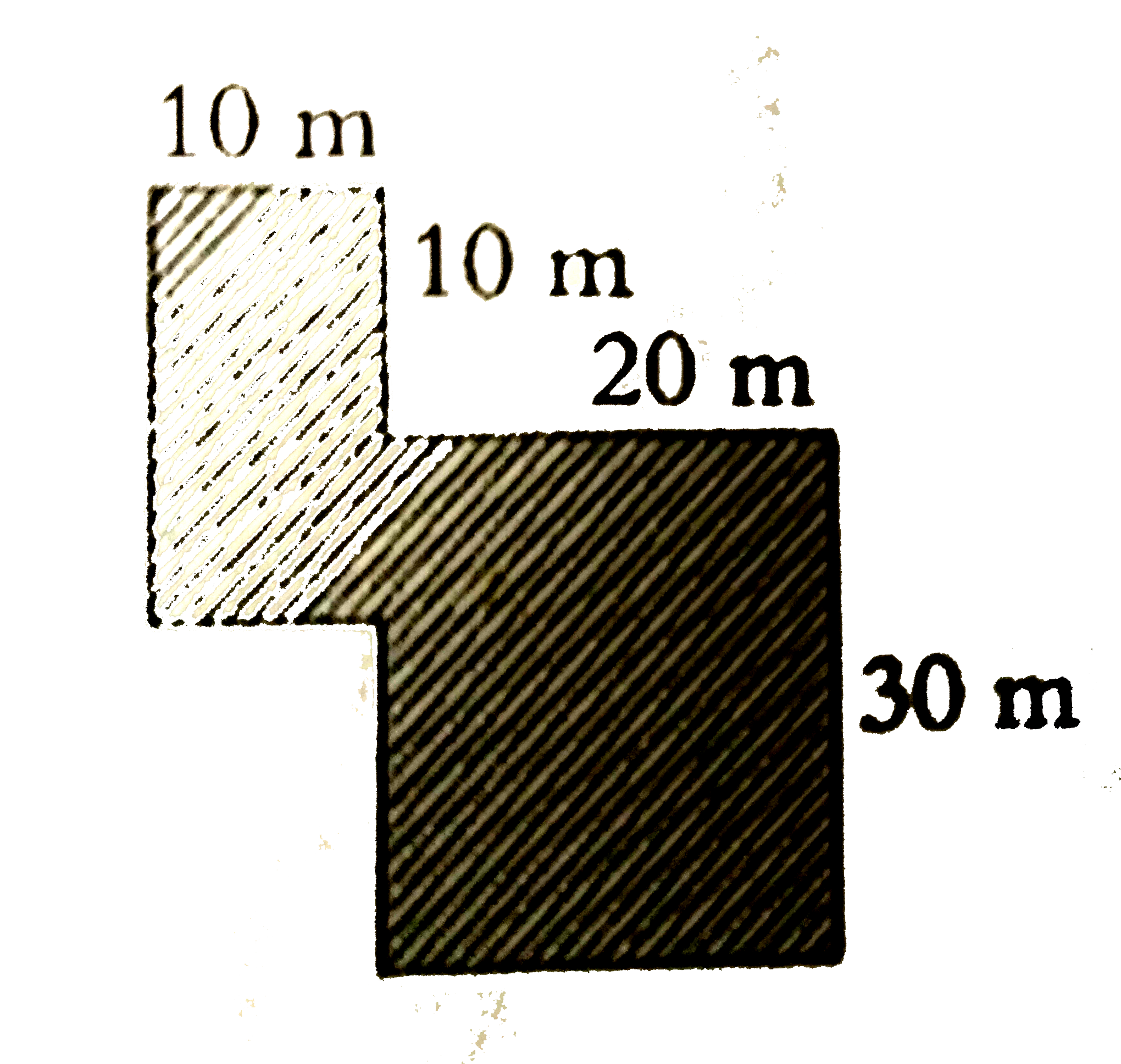
View SolutionFind the area of the figure given below.
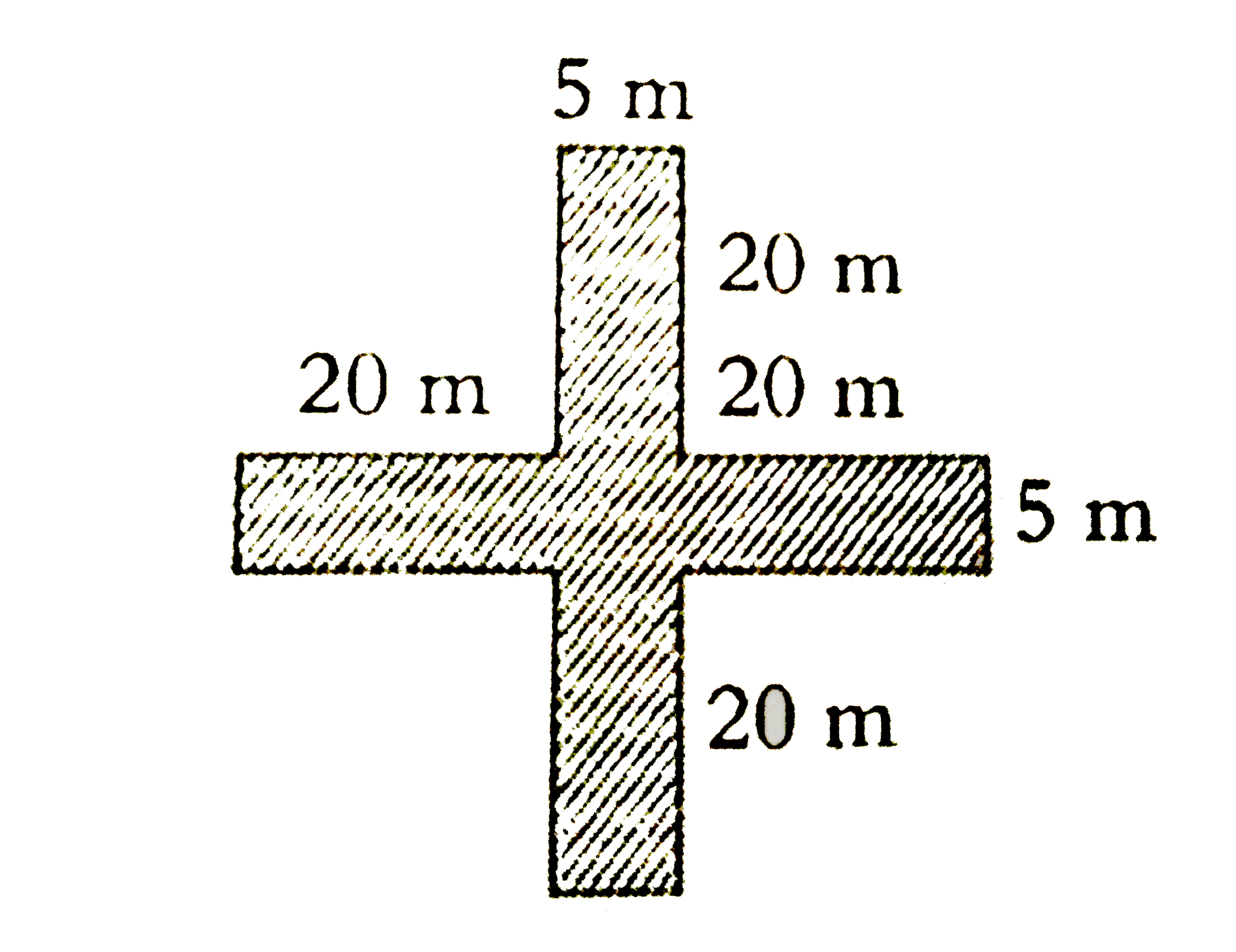
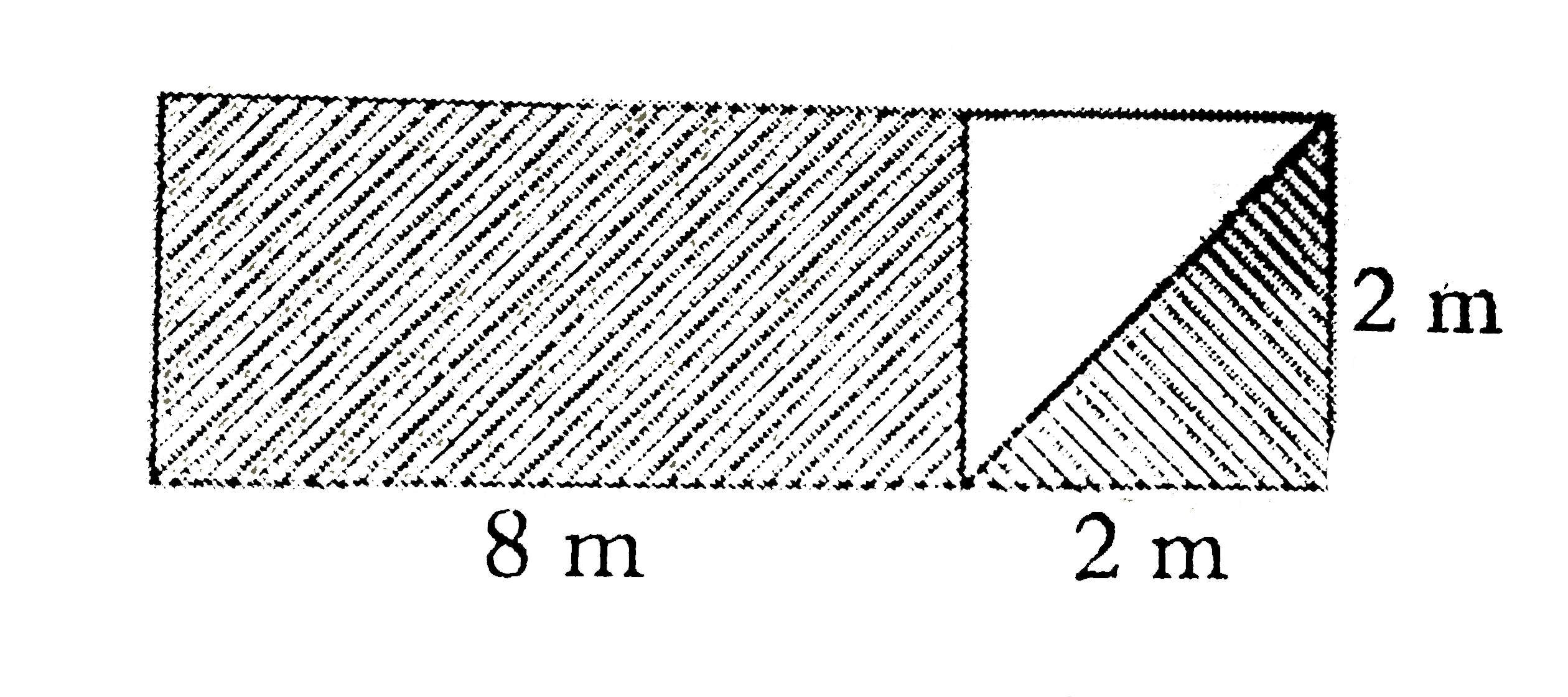
View SolutionThe area of the shaded part in the figure given below is ______.
View SolutionThe inequation represented by the graph given below is :
View Solution

