A monoatomic ideal gas goes through a cyclic process as shown in the figure. The effciency of this process is

0.78
0.62
0.42
0.21
The correct Answer is:A
W=PΔV
WAB=0.
WBC=3P(2V−V)=3PV.
WCD=0.
WDA=−PV.
total work done, W=WAB+WBC+WCD+WDA.
= 0+ 3PV + 0 - PV.
= 2PV.
Heat transfer from system A to B = nCVΔT.
=n32RΔT(∵CV⇒32R).
= 32×V×ΔP .
=32×V×(3P−P) .
= 32×V×(2P) .
= 3VP.
similarly, heat transfer from system B to C = nCPΔT .
=n(52R)ΔT .
52×(3P)(2V−V).
=52×3P×V .
15 PV/2 .
The heat is released from C to D and D to A. .
Efficiency,(η)=Workdo≠bygasHeatgiven→gas×100 .
= 2PV3PV+152PV×100 .
= 2PVPV(3+152)×100.
=26+152×100.
∴η=421×100=19.04%

Topper's Solved these Questions
- EAMCET QUESTION PAPER 2017Book:SIA PUBLICATIONChapter:EAMCET QUESTION PAPER 2017Exercise:CHEMISTRY
Explore 6 Videos
- EAMCET QUESTION PAPER 2017Book:SIA PUBLICATIONChapter:EAMCET QUESTION PAPER 2017Exercise:CHEMISTRY
Explore 6 Videos
- EAMCET -2016 (TS)Book:SIA PUBLICATIONChapter:EAMCET -2016 (TS)Exercise:CHEMISTRY
Explore 36 Videos
- EAMCET-2019 (TS) SHIFT-1Book:SIA PUBLICATIONChapter:EAMCET-2019 (TS) SHIFT-1Exercise:CHEMISTRY
Explore 10 Videos
Similar Questions
The Lewis structure for O3 molecule is given below. The correct formal charges on oxygen atoms labelled 1,2,3 are respectively

View SolutionThe Lewis structure for O3 molecule is given below. The correct formal charges on oxygen atoms labelled 1,2,3 are respectively

View SolutionAssertion (A) Atoms with completely filled and half-filled subshells are stable.
Reason (R ) Completely filled and half filled subshells have symmetrical distribution of electrons and have maximum exchange energy.
The correct answers is
View SolutionFrom the following energy level of hydrogen atom, the values of E∞ and E3 in J are , respectively

View SolutionHow many lines of symmetry does the above figure have ?
View SolutionDetermine the point of symmetry of a regular hexagon.
View SolutionDtermine the images of the following figure about the given line :
View SolutionMatch the following Column A to Column B

View SolutionMatch the following Column A to Column B
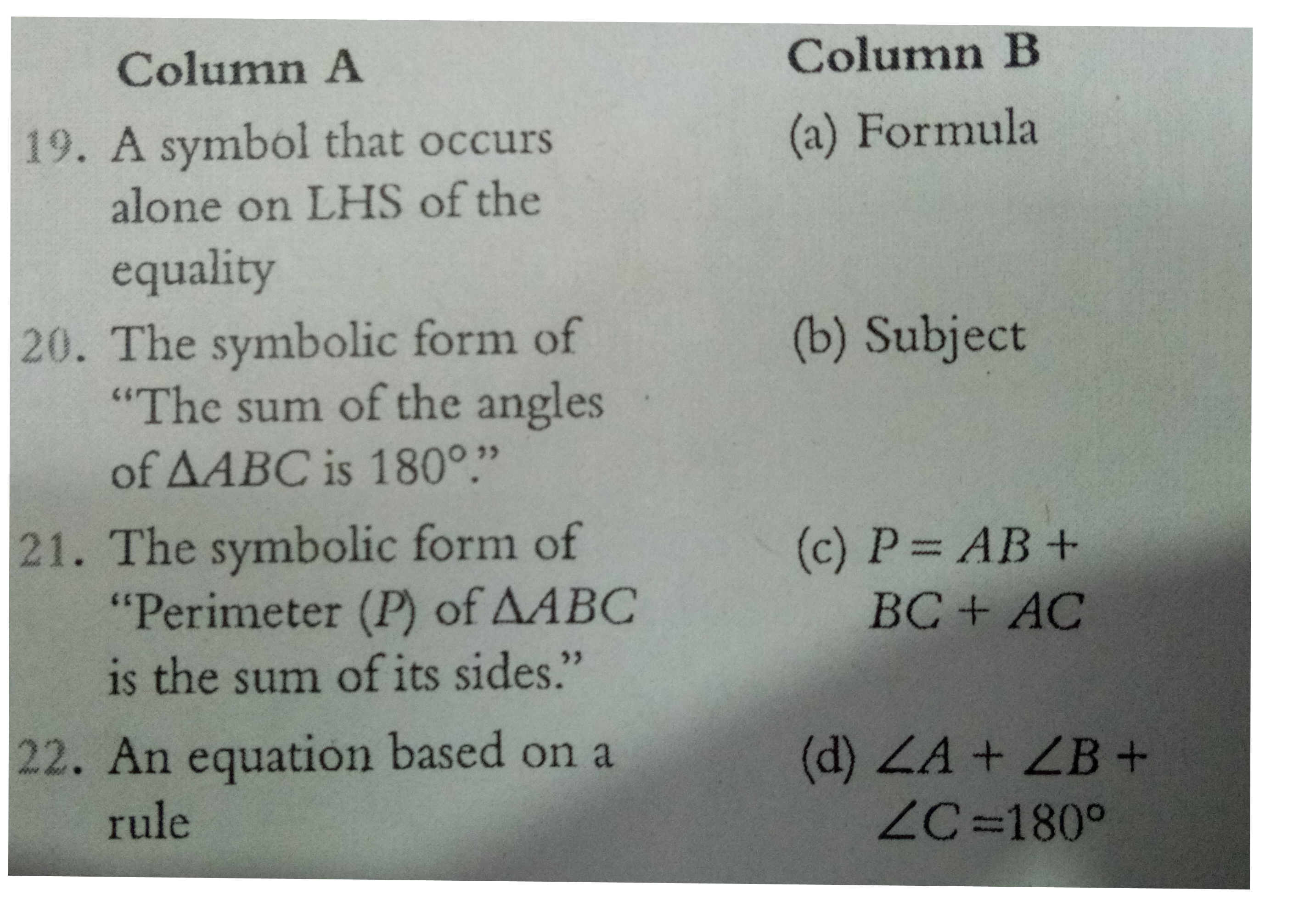
View SolutionMatch the following Column A to Column B

View SolutionThe inequation represented by the graph given below is :
View SolutionThe inequation that best describes the graph given below is _________
View SolutionThe inequation that best describes the following graph is _________
View SolutionWhich inequations represent the shaded region in the given figure
View SolutionThe equation of straight line shown in figure is :
ltimg src="https://d10lpgp6xz60nq.cloudfront.net/physics_images/ALN_PHY_R04_E01_020_Q01.png" width="80%"gt
View SolutionWhat is the distance OA for the square shown in figure :- ltimg src="https://d10lpgp6xz60nq.cloudfront.net/physics_images/ALN_PHY_R05_E01_015_Q01.png" width="80%"gt
View Solution

