1 mol of an ideal gas is enclosed in a cylinder fitted with a frictionless and weightless piston. The gas absorbs x kJ heat and undergoes expansion. If the amount of expansion work done by the gas is x kJ, then the expansion is-Solution in Bengali
adiabatic
cyclic
isothermal
cannot be predicted
The correct Answer is:C
Step by step video, text & image solution for 1 mol of an ideal gas is enclosed in a cylinder fitted with a frictionless and weightless piston. The gas absorbs x kJ heat and undergoes expansion. If the amount of expansion work done by the gas is x kJ, then the expansion is- by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
What is the work done in free expansion of an ideal gas?
View Solution550 kJ "cycle" ^(-1) work is done by 1 mol of an ideal gas in a cyclic process. The amount of heat absorbed by the system in one cycle is
View SolutionA gas undergoes expansion according to the following graph. Calculate work done by the gas
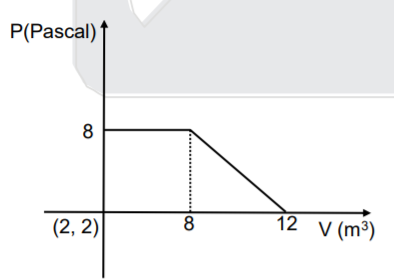
View SolutionA gas undergoes expansion according to the following graph. Calculate work done by the gas

View SolutionAssertion : In an isothemal expansion the gas absorbs heat and does work . Reason: In an isothermal process there is no change in internal energy of an ideal gas.
View SolutionWhether in isothermal expansion of an ideal gas the work done by the gas equals the heat absorbed ?
View SolutionSlow compression or expansion of a gas contained in a cylinder fitted with frictionless piston is_____process.
View SolutionAn ideal gas enclosed in a cylinder fitted with a frictionless and weightless piston, pushes it outside. What would be the sign convention for the work ?
View Solution
