Identify the Lewis acids and Lewis bases in the reaction below:
CO2+OH−→HCO−3Solution in Bengali
Accordong to Lewis concept, an acid is a substance which can accept a pair of electrons. Generally, cotaions (e.g., Ag+,H+,K+, etc), molecules with the central atom having incomplete octet (e.g., SiF4,AlF3,RMgX,BF3, etc.) and molecules in which the central atom is linked to an electronegative atom through double bonds (e.g., CO2) can act as Lewis acid. in the given reactions. Lewis acids are: H+,Co3+,BF3,CO2,AlF3.
According to Lewis concept, a base is a substance which can donate a pair of electron. anions (e.g., F−,OH−, etc.) and molecules having unshared electron pairs act as Lewis base. in the given reactions, Lewis bases are OH−,..NH3,F−.
Similar Questions
Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base: (1) OH^(-) (2) F^(-) (3) H^(+) (4) BCl_(3) .
View SolutionIdentify the lewis acid and the lewis base in the following reactions. Cr3++6H2O→[Cr(H2O)6]3+
View SolutionIdentify the lewis acid and the lewis base in the following reactions. CaO+CO2→CaCO3
(ii) CH3−O−CH3+AlCl3→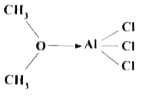
View SolutionBF_(3) and NH_(3) undergo a lewis aciid-base reaction forming an adduct. Which picture below correctly represents the curved arrow notation for the initial lewis acid-lewis base interaction in this reaction, what is the lewis acid and the lewis base?
View SolutionExplain Lewis acid base theory with suitable example. Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base.
a. OH− b. F− c. H+ d. BCl3
View SolutionClassify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/ base: (a) 'OH'- (b) F'- (c) 'H^+(d) BCl_3' ?
View SolutionClassify the following species into Lewis acis and Lewis bases and show how these act as Lewis acbase:OH−
View SolutionFor each of the following reaction, identify the Lewis acid and the Lewis base.
CO2OH−→HCO−3
View Solution
