Balance the equation by ion-electron method :
Zn+NaNO3+NaOH→Na2ZnO2+NH3+H2OSolution in Bengali
Step by step video & image solution for Balance the equation by ion-electron method : Zn+ NaNO_(3) + NaOH to Na_(2)Zn O_(2) + NH_(3) + H_(2)O by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Balance the following chemical reaction by oxidation number method :
NaNO3+Zn+NaOH→NH3+Na2ZnO2+H2O
View SolutionBalanced equation [Zn+H^(+)+NO_(3)^(-)toNH_(4)^(+)+Zn^(+2)+H_(2)O] in NH_(4)^(+) The coefficient of is:
View SolutionBalance the following equations by oxidation number method.
Zn+HNO3→Zn(NO3)2+H2O+NH4NO3
View SolutionBalance the following equations by ion electron method: a. CuO + NH_(3) rarr Cu+H_(2)O+N_(2)
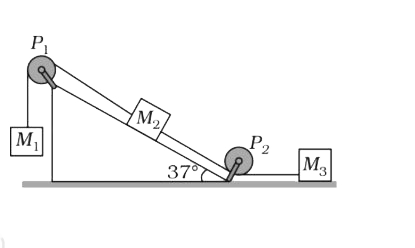
View SolutionMasses M1,M2 and M3 are connected by strings of negligible mass which pass over massless and frictionless pulleys P1 and P2 as shown in the figure. The masses move such that the portion of the string betweenP1 and P2 is parallel to the inclined plane and the portion of the string between P2 and M3 is horizontal. The masses M2 and M3 are 4.0 kg each and the coefficient of kinetic friction between both the masses and the surfaces is 0.25. The inclined plane makes an angle of 37° with the horizontal. If the mass M1 moves downwards with a uniform velocity, find
(i) the mass of M1 in kg
(ii) the tension in the horizontal portion of the string in Newton
View SolutionIn the balanced equation -
[Zn+H++NO−3→NH+4+Zn+2+H2O]
coefficient of NH+4 is :-
View Solutionआयन-इलेक्ट्रॉन विधि द्वारा निम्नलिखित समीकरणों को संतुलित करें-
Zn+NO−3→Zn+++NO2 (acidic solution)
View Solutionin the reaction, Zn+NaNO_(3)+NaOHtoNa_(2)ZnO_(2)+A+H_(2)O With the simplest integer coefficients, the sum of the stoichiometric coefficients of Zn and A in a balanced reaction is:
View Solution

