The rate constants of some reactions are given below :
(i) k=1.2×10−3dm3.mol−1.s−1
(ii) k=5.2×10−2atm3.s−1
(iii) k=3.2×10−3dm3/2.mol−1/2.s−1
(iv) k=4.5×10−3dm6.mol−2.s−1
Reactions with the same overall order are - Solution in Bengali
(i)
(ii)
(iii)
(iv)
The correct Answer is:B, D
Step by step video, text & image solution for The rate constants of some reactions are given below : (i) k=1.2xx10^(-3)"dm"^(3)."mol"^(-1).s^(-1) (ii) k=5.2xx10^(-2)"atm"^(3).s^(-1) (iii) k=3.2xx10^(-3)"dm"^(3//2)."mol"^(-1//2).s^(-1) (iv) k=4.5xx10^(-3)"dm"^(6)."mol"^(-2).s^(-1) Reactions with the same overall order are - by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL KINETICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL KINETICSExercise:EXERCISE (VERY SHORT ANSWER -TYPE QUESTIONS)
Explore 19 Videos
- CHEMICAL KINETICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL KINETICSExercise:EXERCISE (Fill in the blanks)
Explore 22 Videos
- CHEMICAL KINETICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL KINETICSExercise:MCQ HOTSPOT (Single Correct Type )
Explore 45 Videos
- BIOMOLECULESBook:CHHAYA PUBLICATIONChapter:BIOMOLECULESExercise:Practic Set 29
Explore 1 Video
- CHEMICAL THERMODYNAMICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL THERMODYNAMICSExercise:Practice Set 6
Explore 15 Videos
Similar Questions
Give two examples each of very fast and very slow reactions.
View SolutionThe rate of a reaction is directly proportional to the ………….
View SolutionAt a particular time , in the reaction 3A+2B→4C -
View SolutionWrite down the structure of tripeptide Gly-Ala-Val.
View SolutionThe condition for spontaneity of process is
View SolutionIn a first order reaction, the concentration of the reactant decreases form 800mol dm−3 to 50mol dm−3 in 2×104s. The rate constant of the reaction (in s−1) is
View SolutionConsider the reaction.
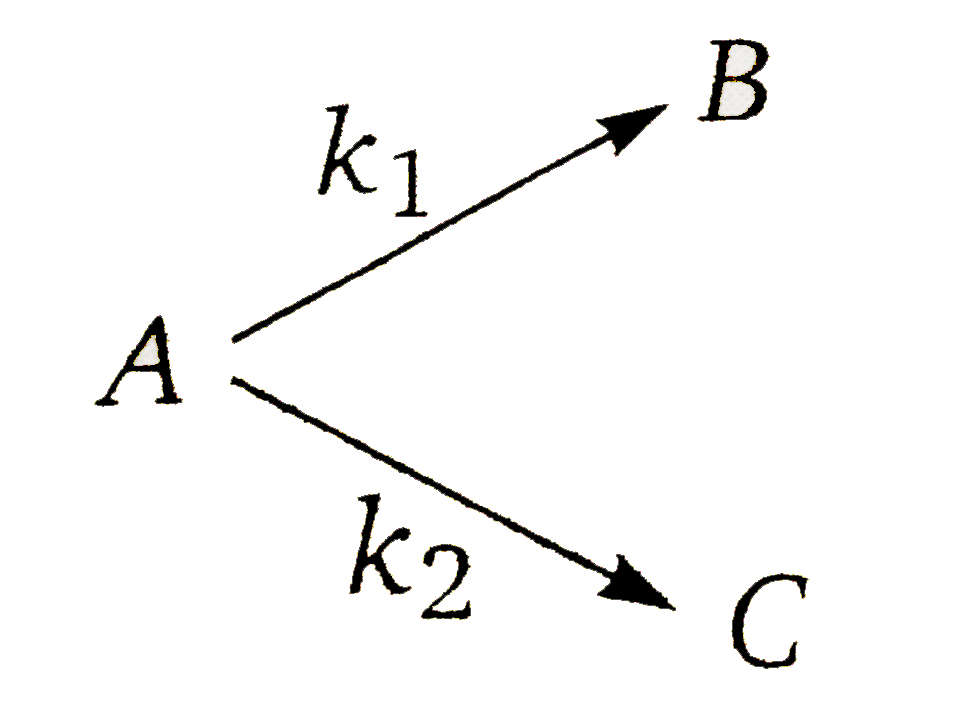
The rate constant for two parallel reactions were found to be 10−2dm−2mol−1s−1 and 4×10−2dm−3mol−1s−1 . If the corresponding energies of activation of the parallel reaction are 100 and 120 kJ/mol respectively, what is the net energy of activation (Ea) of A
View SolutionFor a hypothetical reaction, the following kinetic data suggested that the overall order of this reaction is
[A][B][C]mol dm−3mol dm−3mol dm−3s−12.01.01.02.04.02.01.04.01.0
View SolutionThe rate constant of a reaction is 10.8×10−5 moldm−3s−1. The order of the reaction is
View SolutionThe rate constant of a reaction is 1.5xx10^(-3)"dm"^(3)."mol"^(-1).s^(-1). Determine its order .
View SolutionThe unit of rate constant of a reaction is (mol)−1/2.(dm)3/2.s−1 . Determine the order of the reaction.
View SolutionFor a chemical raction A→B the rate of the reaction is 2×10−3 mol dm−3s−1, when the initial concentration is 0.05 mol dm−3. The rate of the same reaction is 1.6×10−2 mol dm−3s−1 when the initial concentration is 0.1 mol dm−3. The order of the reaction is
View SolutionFor a chemical raction A→B the rate of the reaction is 2×10−3 mol dm−3s−1, when the initial concentration is 0.05 mol dm−3. The rate of the same reaction is 1.6×10−2 mol dm−3s−1 when the initial concentration is 0.1 mol dm−3. The order of the reaction is
View SolutionFor a chemical raction A→B the rate of the reaction is 2×10−3 mol dm−3s−1, when the initial concentration is 0.05 mol dm−3. The rate of the same reaction is 1.6×10−2 mol dm−3s−1 when the initial concentration is 0.1 mol dm−3. The order of the reaction is
View SolutionIf the rate constant of reaction k=2×10−6s−1atm−1 . Its value in dm3mol−1s−1 is
View Solution
