plot a graph of reaction -rate vs concentration of the reactant for a fist order reaction. Solution in Bengali
Step by step video & image solution for plot a graph of reaction -rate vs concentration of the reactant for a fist order reaction. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Plot of rate of reaction against the concentration of the reactant gives a straight line passing through the origin. The order of the reaction is ...........
View SolutionExplain with an example, what is a pseudo first order raction ? The graphs (A and B) given below are plots of rate of reaction Vs concentration of the reactant. Predict the order from the graphs.
View SolutionPlot the graph of t t_(1//2) vs concentration for first order and zero order reaction.
View SolutionFor a reaction the graph of the rate of the reaction against molar concentration of the reactant is as shown :
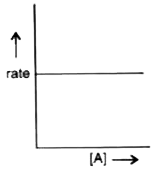
What is the order of the reaction ?
View SolutionWhen rate of a 1st order reaction is plotted Vs. concentration of the reactant a straight line is obtained, whose slope is equal to____
View SolutionA plot of reactant concentration versus time for a reaction is a straight line with a negative slope giving the rate constant, and the intercept, giving the initial concentration of the reactant. The order of the reaction is
View SolutionA plot of logarithm of rate vs logarithm of concentration of the reactant in a first order reaction is straight line whose slope is tanθ, where θ is
View Solution
