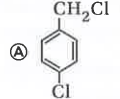
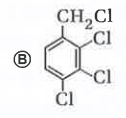
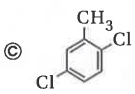
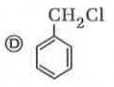
An aromatic compound C7H6Cl2(A) , gives AgCl on boiling with alcoholic AgNO2 solution and yields C7H7OCl on treatment with sodium hydroxide. A on oxidation gives monochlorobezoic acid. The compound A is-Solution in Bengali
The correct Answer is:A
Similar Questions
An aromatic compound 'A' (molecular formula C_(8)H_(8)O ) gives positive 2, 4-DNP test . It gives a yellow precipitate of compound 'B' on treatment with iodine and sodium hydroxide solution. Compound 'A' does not give tollen's or fehling's test. On drastic oxidation with potessium permanganate it forms a carboxylic acid 'C' (Molecular formula C_(7)H_(6)O_(2) ), which is also formed along with the yellow compound in the above reaction. identify A,B and C and write all the reactions involved.
View SolutionCompound (A) of molecular formula C7H8 when treated with air in presence of V2O5 at 773 K gives a compound (B) of molecular formula C7H6O, which has the smell of bitter almonds. Alkaline KMnO4 oxidises compound (B) to ( C) of molecular formula C7H6O2. Compound (B) on treatment with N2H4 and KOH gives back compound (A). Identify (A), (B) & ( C) and explain the reactions.
View SolutionAn organic compound (A) of molecular formula C_(7)H_(6)O is called as oil of bitter almonds. (A) on oxidation gives (B) of molecular formula C_(7)H_(6)O_(2) which gives brisk effervescence with aqueous alcoholic KCN, compound ( C) is formed. Identify A, B and C and write the equations.
View Solutionअणु - सूत्र C7H8O वाला एक यौगिक जल में तथा तनु सोडियम बाइकार्बोनेट में अविलेय है परन्तु तनु जलीय सोडियम हाइड्रोक्साइड में विलेय है| यह यौगिक जलीय FeCl3 के साथ विशिष्ट रंग देता है| तथा ब्रोमीन जल के साथ अभिकृत करने पर तेजी के साथ C7H5OBr3 का अवक्षेप देता है| यौगिक की संरचना लिखिए |
View SolutionAn organic compound (A) of molecular formula C7H8O on oxidation with alkaline KMnO4 gives (B) of formula C7H6O. (B) on reaction with Cl_(2) in the presence of catalyst FeCl3 gives ( C ) of formula C7H5OCl. .(B)onreactionwithCl_(2)∈the|e|nceofcatalystgivesC_(7)H_(5)OCl. Identify A, B, C, D and explain the reaction involved.
View SolutionAn aromatic compound X, C7H7Cl on oxidation gives an aromatic compound Y. The sodalime decarboxylation of Y produces benzene. X is :
View SolutionAn aromatic compound C7H6Cl2 (A) gives AgCl on boiling with alcoholic AgNO3 solution and yeilds C7H7Ocl on treatement with sodium hydroxide (A) on oxidation gives monochlorobenzoic acid. The compound (A).
View Solution


