[H_(2)" gas undergoes process where "PV^(2)=" constant.The ratio of work done by gas to modulus of change in "],[" its internal energy as the gas expands is "(2)/(N).N" is equal to "]
Step by step video & image solution for [H_(2)" gas undergoes process where "PV^(2)=" constant.The ratio of work done by gas to modulus of change in "],[" its internal energy as the gas expands is "(2)/(N).N" is equal to "] by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
A certain amount of mono - atomic ideal gas undergoes a process ρUn= constant where ρ is density of gas and U is internal energy of the gas.It is found that the ratio WδU for this process is 23. Find the value of n.
View SolutionIn the process pV2= constant, if temperature of gas is increased, then
View Solution
Knowledge Check
An ideal monatomic gas undergoes process PV1.25 = constant .Then
Aupon increase in pressure temperature decreasesBupon increase in pressure heat is absorbed by gasCif heat is given to gas its internal energy increasesDheat is absorbed by gas if volume increasesIn an adiabatic process 90 J of work is done on the gas. The change in internal energy of the gas is
A−90jB+90jC0 jDDepends on initial temperatureA monoatomic gas follow a process TV= constant. Calculate the ratio of work done to change in internal energy when the gas goes form one state to another.
A23B−23C53D75
An ideal monoatomic gas undergoes a process in which its internal energy U and density ρ vary as Uρ= constant. The ratio of change in internal energy and the work done by the gas is
View SolutionA gas undergoes a process in which its pressure P and volume V are related as VPn= constant. The bulk modulus of the gas in the process is:
View SolutionAn ideal gas undergoes cyclic process ACBC as shown is given PV diagram
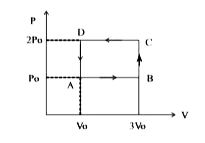
The amount of work done by the gas is
View SolutionIn an adiabatic expansion of 2 moles of a gas, the change in its internal energy was found to be –100J. The work done in this process is :
View SolutionAn ideal gas with adiabatic exponent (γ=1.5) undergoes a process in which work done by the gas is same as increase in internal energy of the gas. Here R is gas constant. The molar heat capacity C of gas for the process is:
View Solution
