Which of the following will be obtained in very small amount during this reaction?
CH3−CH=CH−CH2−C∣∣O−O−H(i)AgOH−−−−−−−−−−−→(ii)Br2/CCl4,ΔProducts
A

B
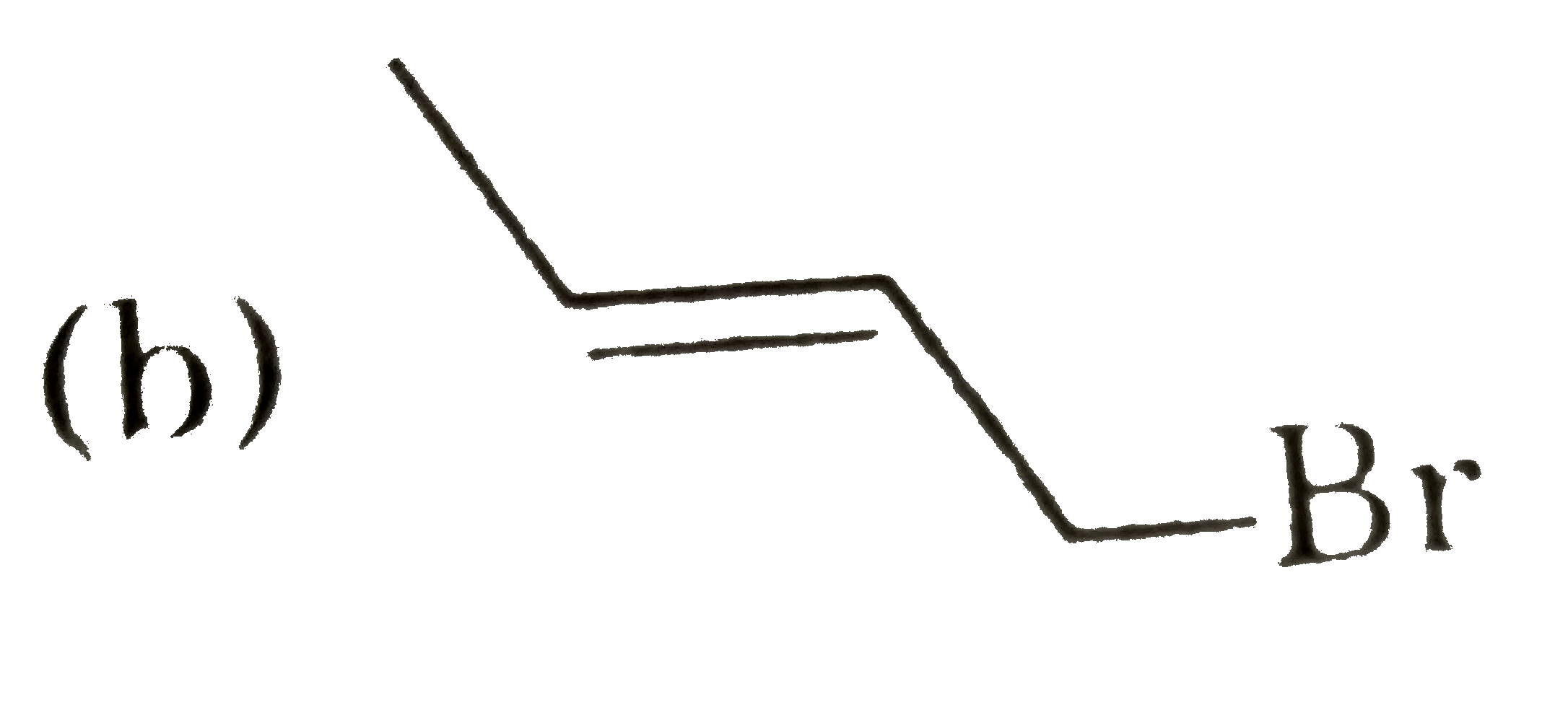
C
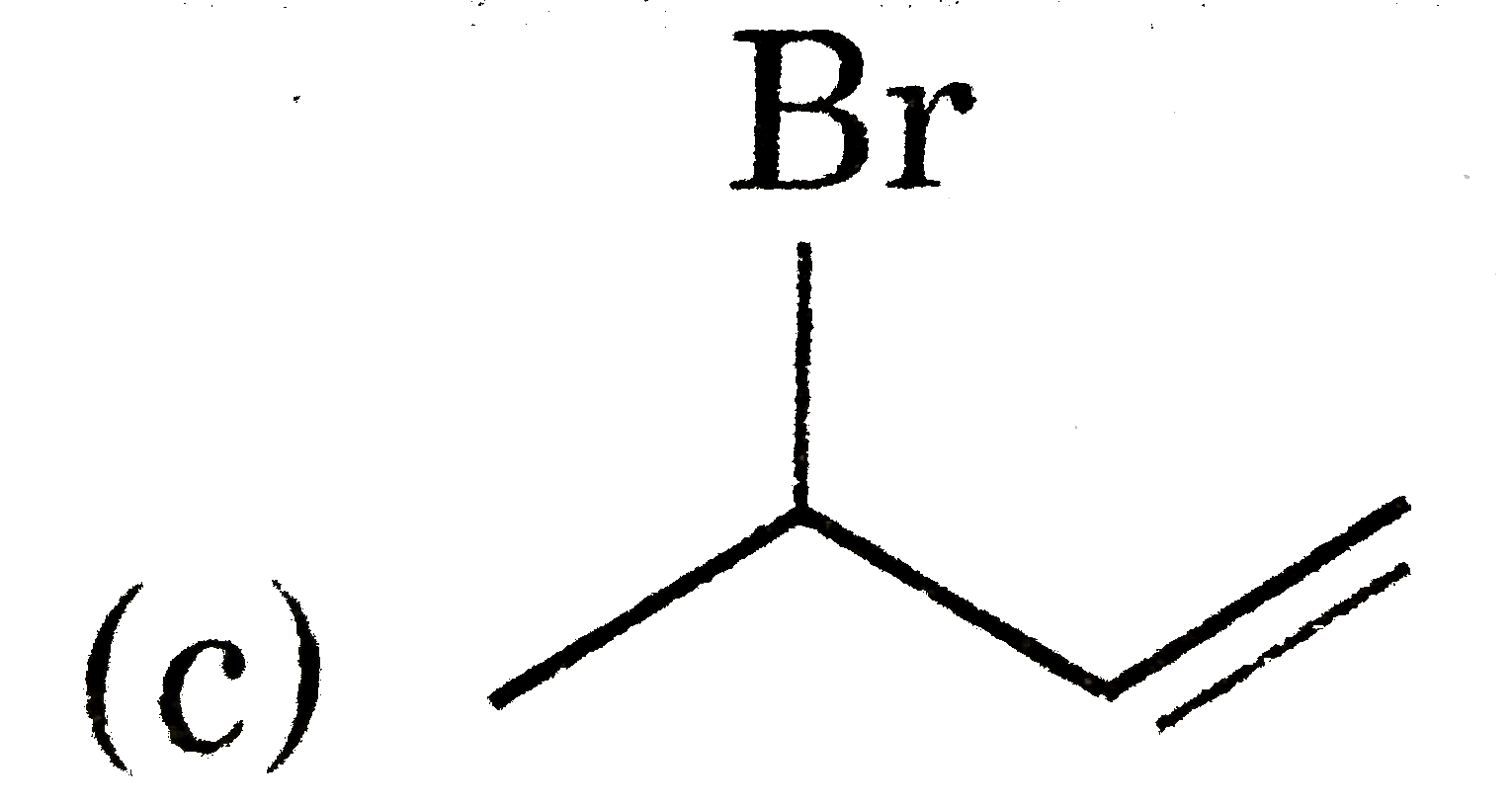
D
CH3−CH=CH−CH2−C−O−CH2 ∣| | O CH=CH−CH3
Text Solution
The correct Answer is:A, B, C, D
|
Topper's Solved these Questions
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSEXERCISE - Comrehnsion
20 Videos
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSEXERCISE - Match the column
14 Videos
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSEXERCISE - REASONING TYPE
9 Videos
- BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEBOOK - GRB PUBLICATIONCHAPTER - BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEEXERCISE - SUBJECTIVE TYPE
40 Videos
- CHEMICAL BONDING-IBOOK - GRB PUBLICATIONCHAPTER - CHEMICAL BONDING-IEXERCISE - Subjective Type
120 Videos
Similar Questions
Knowledge Check
H3C−CH3∣C∣CH3−H+CCl4R2O2−−→hvProduct :
AH3C−CH3∣C∣CH3−ClBCHCl3CBoth (a) and (b)DNone of theseCH_(3)-underset(Br)underset(|)CH-CH_(2)-overset(O)overset(||)C-CH_(3)underset(Delta)overset(N_(2)H_(4),overset(Ө)OH)rarr product :
A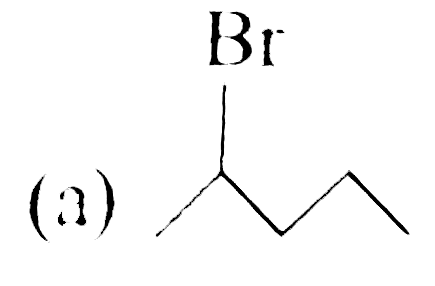 B
B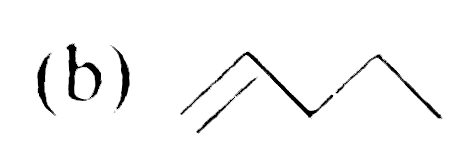 C
C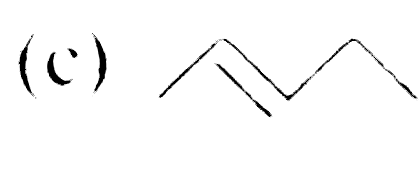 D
D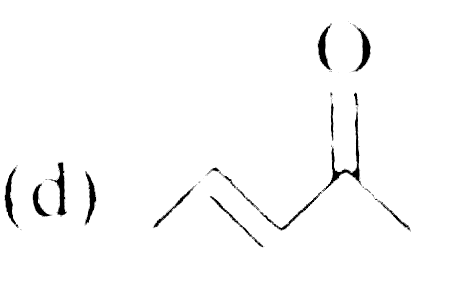
2CH3−C∣∣O−CH3Mg/Hg−−−−→H+ Product in the reaction is
AH3C−CH3∣C∣OH−CH3∣C∣OH−CH3BCH3−C∣∣O−O−C∣∣O−CH3CCH3−CH∣OH−CH∣OH−CH3DNone of these
CH3−CH2−O−C∣CH3=CH2H3O⊕−−−→Products are
View SolutionWhich of the following is true regarding increasing rectvityy in the E2 reaction wioth Na+C2H5O−?
CH3−CH3∣C∣CH3(A)−Br D3C−CD3∣C∣CD3(B)−Cl H3C−CH3∣C∣CH3(C)−Cl
View SolutionIn the following reaction :
H3C−CH3| C | CH3−CH=CH2H2O/H⊕−−−−−→A
The major product A is :
View SolutionCH_(3)-underset(Br)underset(|)(CH)-CH_(2)-overset(O)overset(||)(C)-CH_(3)underset(Delta)overset(N_(2)H_O(4),overset(ϴ)(O)H)(to) Product:
View SolutionThe major product formed in the reaction is:
CH3−CH3∣C∣H−CH2BrCH3OӨ−−−−→CH3OH
View Solution

