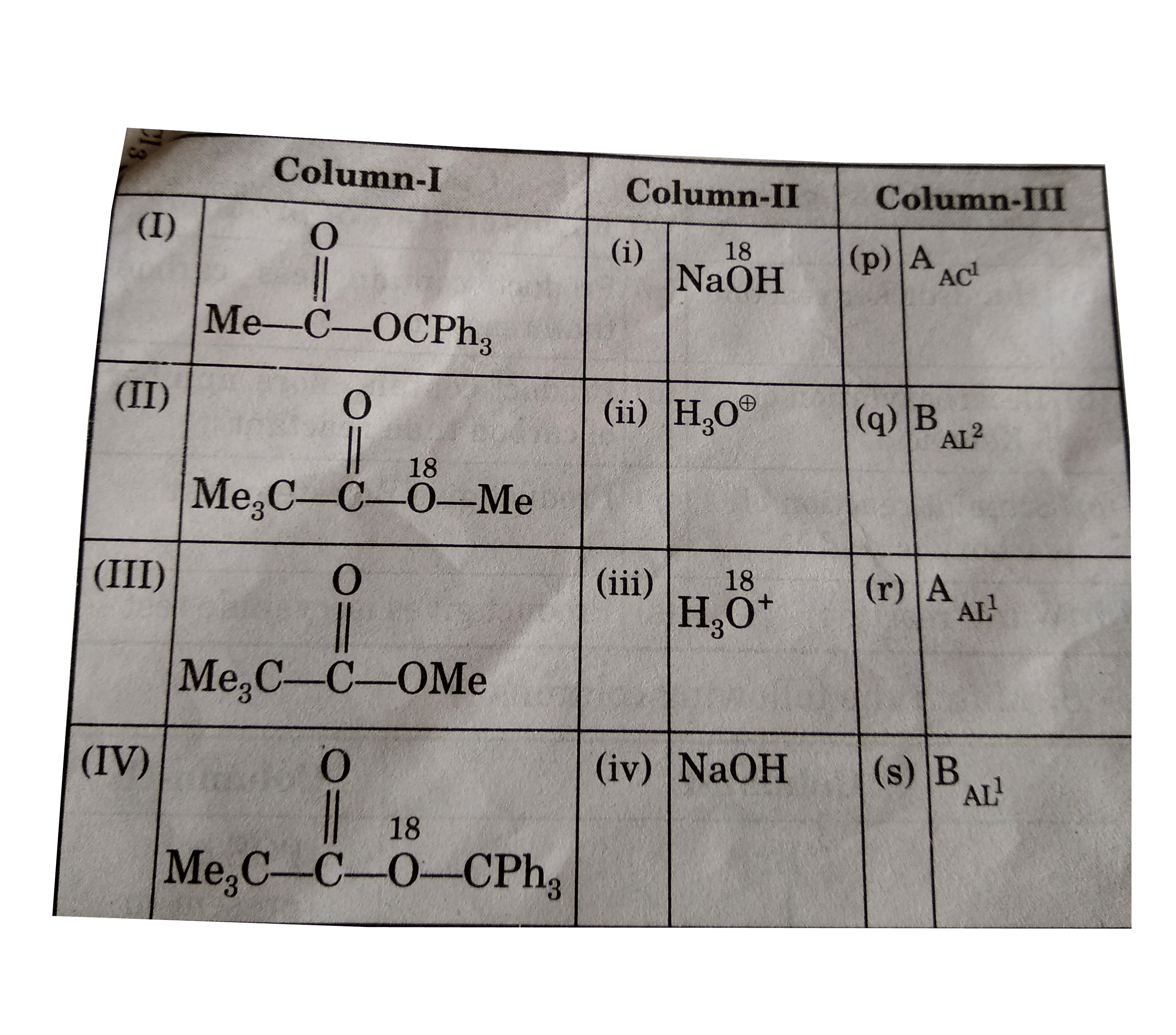
Combination in which Ph3C−OH will be formed
A
IV, ii, r
B
IV, i, q
C
I, iii, r
D
I, I, s
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSEXERCISE - Match the column
14 Videos
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSEXERCISE - Subjective type
19 Videos
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSEXERCISE - MULTIPLE OBJECTIVE TYPE
48 Videos
- BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEBOOK - GRB PUBLICATIONCHAPTER - BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEEXERCISE - SUBJECTIVE TYPE
40 Videos
- CHEMICAL BONDING-IBOOK - GRB PUBLICATIONCHAPTER - CHEMICAL BONDING-IEXERCISE - Subjective Type
120 Videos
Similar Questions
Knowledge Check
PH3 can be formed in
Aheating hypophosphorus acidBheating orthophosphorus acidCreacting white phosphorus with hot conc. NaOHDallFour hydroxy compounds have functional groups as shown
X:−CH2OH,Y:−∣CHOH
Z:Ph−OH,W:Ph−C∣HOH
The purple colouration with FeCl3 will be given byAX onlyBX and YCZ onlyDX, Y, Z and WReaction of acetaldehyde with NH2OH will be fastest at which of the following pH?
A1B7C8D6
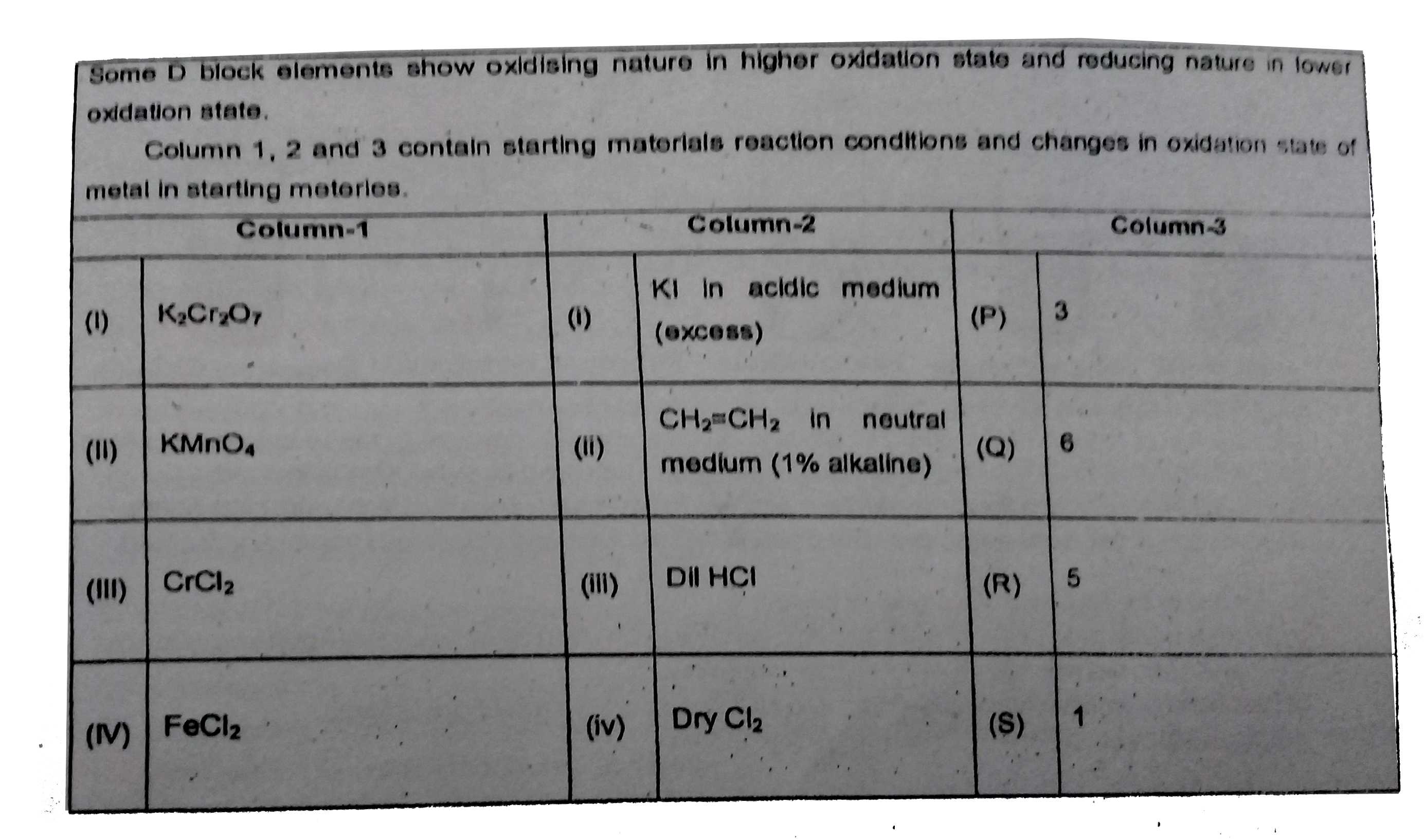
Select the appropriate combination which gives glycol ⎡⎢⎣C∣OHH2−C∣OHH2⎤⎥⎦ as a product.
View SolutionDissociation constant of CH3COOH and NH4OH in squeous solution are 10−5 if pH of a CH3COOH solution is 3, what will be the pH of NH4OH ?
View SolutionThe deamination of Ph2C(OH)CH2NH2 with NaNO2−HCl gives a product (P), which on oxidation gives benzonic acid only. Identify the product (P)
View SolutionThe product of the reaction of Ph2CHCh2OH with HBr is
View SolutionDegree of dissocation of CH3COOH and NH4OH are the same. If 0.1M solution of CH3COOH has pH=4.0, them pH of 0.01MNH4OH will be
View Solution

