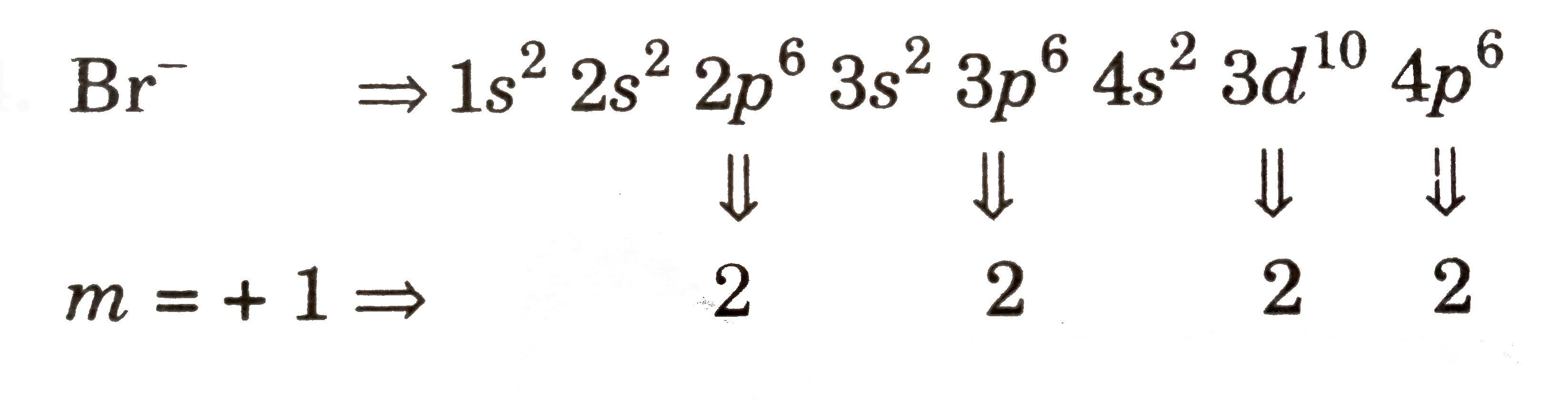Find the number of electrons are there in NI2+ ion which are having the (+1) value of magnetic quantum number (m) ?
Video Solution
Text Solution
The correct Answer is:8
|
Similar Questions
Knowledge Check
A 3d electron having s=+1/2 can have a magnetic quantum number
A+2B+3C−3D−4If the value of azimuthal quantum number of an electron is 2, then which of the following values of magnetic quantum numbers is not permissible,
A3B2C0D1The maximum number of electrons with spin value +1/2 in the orbital with azimuthal quantum number value 1=2 and magnetic quantum number m=+1 is...
A5B6C3D1
The number of electrons present in the Br− ion that have the value of magnetic quantum number m=+1 is ______.
View Solution" Find the number of electrons in the ground state of "N" -atom having "(n+1+m)" value of "2"
View SolutionThe number of electrons for Zn2+ cation that have the value of azimuthal quantum number =0 is:
View SolutionNumber of two electron can have the same values of … quantum numbers
View SolutionNo two electron can have the same values of ..... quantum numbers.
View Solution