Compound A on hydrolysis gives gaseous compound B and alkaline solution. When B reacts with Nessler's reagent it gives brown ppt. when 'B' reacts with H2[PtCI6] it gives yellow ppt of C. Hybridised state of central atom of the ionisation sphere of compound C is:
sp3
dsp2
sp3d2
d2sp3
The correct Answer is:A
(NH4)2[PtCI6]
Here, N is sp3 hyb
Topper's Solved these Questions
- SALT ANALYSISBOOK - GRB PUBLICATIONCHAPTER - SALT ANALYSISEXERCISE - G: Ist Group Cations
20 Videos
- SALT ANALYSISBOOK - GRB PUBLICATIONCHAPTER - SALT ANALYSISEXERCISE - H: IInd Group Cations
49 Videos
- SALT ANALYSISBOOK - GRB PUBLICATIONCHAPTER - SALT ANALYSISEXERCISE - E.Precipitation/Redox Reacttions
48 Videos
- S BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - S BLOCK ELEMENTSEXERCISE - SUBJECTIVE TYPE
9 Videos
- THERMODYNAMICSBOOK - GRB PUBLICATIONCHAPTER - THERMODYNAMICSEXERCISE - All Questions
704 Videos
Similar Questions
Compound which does not give yellow ppt, when treated with l2 in alkali is
View SolutionA nitraite on acid hydrolysis gives compound A, which reacts with thionyl chloride to give compound B. Benzene reacts with compound B in presence of anhydrous AlCl3 to give compound C. The compound B reacts with ethyl alcohol to give ester D. Identify the compounds A,B C and D and write the equations.
View Solution
Knowledge Check
Which of the following compound does not give yellow ppt. when react with NaOI ?
A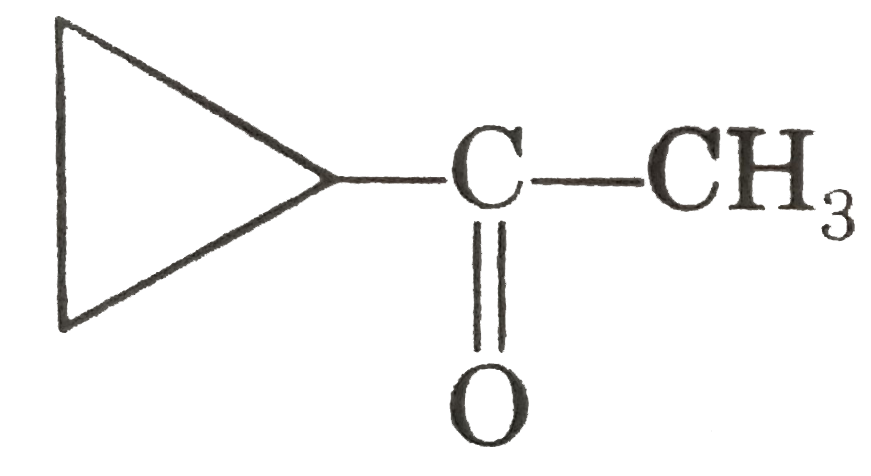 BPh−CH∣OH−CH2CMeCH∣OH−COOHDPh−CH2−C∣∣O−CH3
BPh−CH∣OH−CH2CMeCH∣OH−COOHDPh−CH2−C∣∣O−CH3A chloride salt on addition of alkali solution gives gas B whicch gives brownn ppt. with nessler's regent. What is A, B and C?
ANH4Cl,NH3andHgO⋅Hg(NH2)(NO3)BNH4Cl,NH3andHg(NH3)ClCNH4Cl,NH3andHgO⋅Hg(NH2)ClDNH4Cl,NH3andHgO⋅Hg(NH2)IThe molecular formula of a compound (A) is C3H7NO. The Compound (A) hydrolysis to give an acid (B) and an amine (C ). Amine (C ) reacts with reagent ( X) to give a product insoluble in KOH. (C ) also gives nitrosoamines on reaction with HNO2. Acid (B) reduces Tollen's reagent to give silver mirror.
Compound A isACH3−O∣∣C−N(CH3)2BH−O∣C−N(CH3)2CC2H5−O∣C−N(C6H5)2DC2H5−O∣C−NH2
Compound C4H8CI2(A) on hydrolysis gives a compound C4H8O(B) which reacts with hydroxylamine and does not give any test with Tollens reagent . What are (A) and (B) ?
View SolutionX' compound (C4H8O) decolorises bromine water react with I2 & NaOH it give yellow ppt identify 'X'
View SolutionThe molecular formula of a compound (A) is C3H7NO. The Compound (A) hydrolysis to give an acid (B) and an amine (C ). Amine (C ) reacts with reagent ( X) to give a product insoluble in KOH. (C ) also gives nitrosoamines on reaction with HNO2. Acid (B) reduces Tollen's reagent to give silver mirror.
Reagent X is
View SolutionThe compound 'A' when trated with ceric ammonium nitrate solution gives yellow ppt. the compound 'A' is
View SolutionAn organic compound A reacts with PCl5 to give compound B. Compound B reacts with Na/ether to give n-butane. What are compounds A and B ?
View Solution

