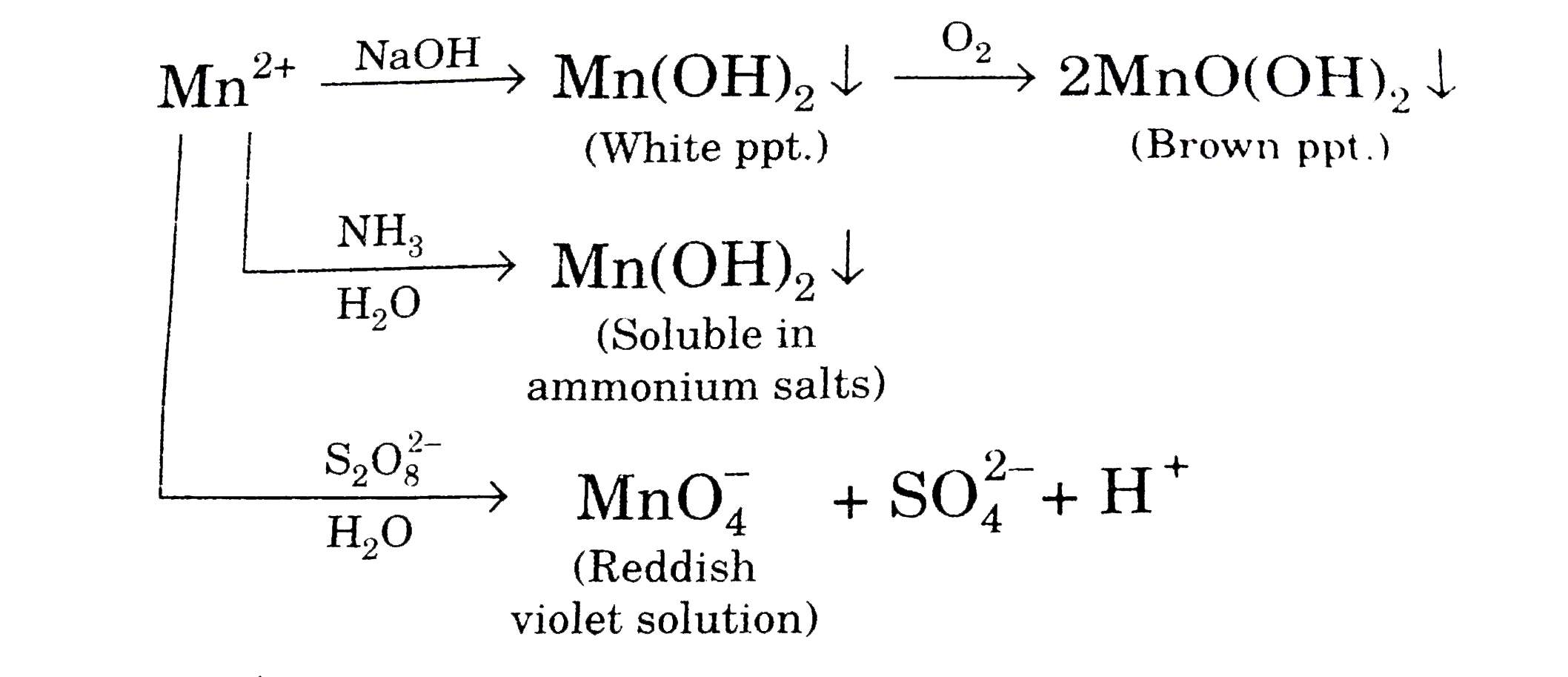A coloured solution of a salt gives following reactions:
I. It gives white precipitate with sodium hydroxide which becomes brown on exposure to air.
II. It gives white precipitate with ammonia solution which is soluble in ammonium salts.
III. Its acidified solution contanining a few drops of dilute AgNO3 on boiling with ammonium peroxodisulphate gives a reddish violet solution. The cation of the salt is:
Mn2+
Zn2+
AI3+
Ni2+
The correct Answer is:A
Similar Questions
Knowledge Check
AgNO3 solution will not give white precipitate with
A[Co(NH3)3Cl3]B[Co(NH3)4Cl2]ClC[Co(NH3)5Cl]Cl2D[Co(NH3)6]Cl3A white salt gives a white precipitate with barium chloride solution which is insoluble in ammonia and hot water. The salt is
ASilver nitrateBMagnesium sulphateCLead AcetateDPotassium chromateWhich of the following reagents give white precipitate with MgSO4 solution, which is readily soluble on addition of ammonium salts?
ANaOHB(NH4)2CO3CNa2CO3DNa2HPO4
A solution of a salt in water, on addition of dilute HCI, gives a white precipitate, which is soluble in hot water. The salt contains
View SolutionConcentrated solution of which salt gives white precipitate with ferric chloride solution:
View SolutionThe aqueous solution of salt gives white ppt with lead aceetate solution which is insolution in but water and nitric acid .The salt contains
View SolutionThe cation which gives white precipitate of its hydroxide when treated with aqueous ammonium sulphide solution is:
View SolutionThe aqueous solution of an unknown sodium salt gives the following reactions
(i) it gives white turbidity with dilute HCl solution (ii) it decolourises a solution of iodine in potassium iodide
(iii) it gives a white precipitate with AgNO3 solution which changes colours and finally becomes black on standing.
The unknown sodium salt is
View Solution