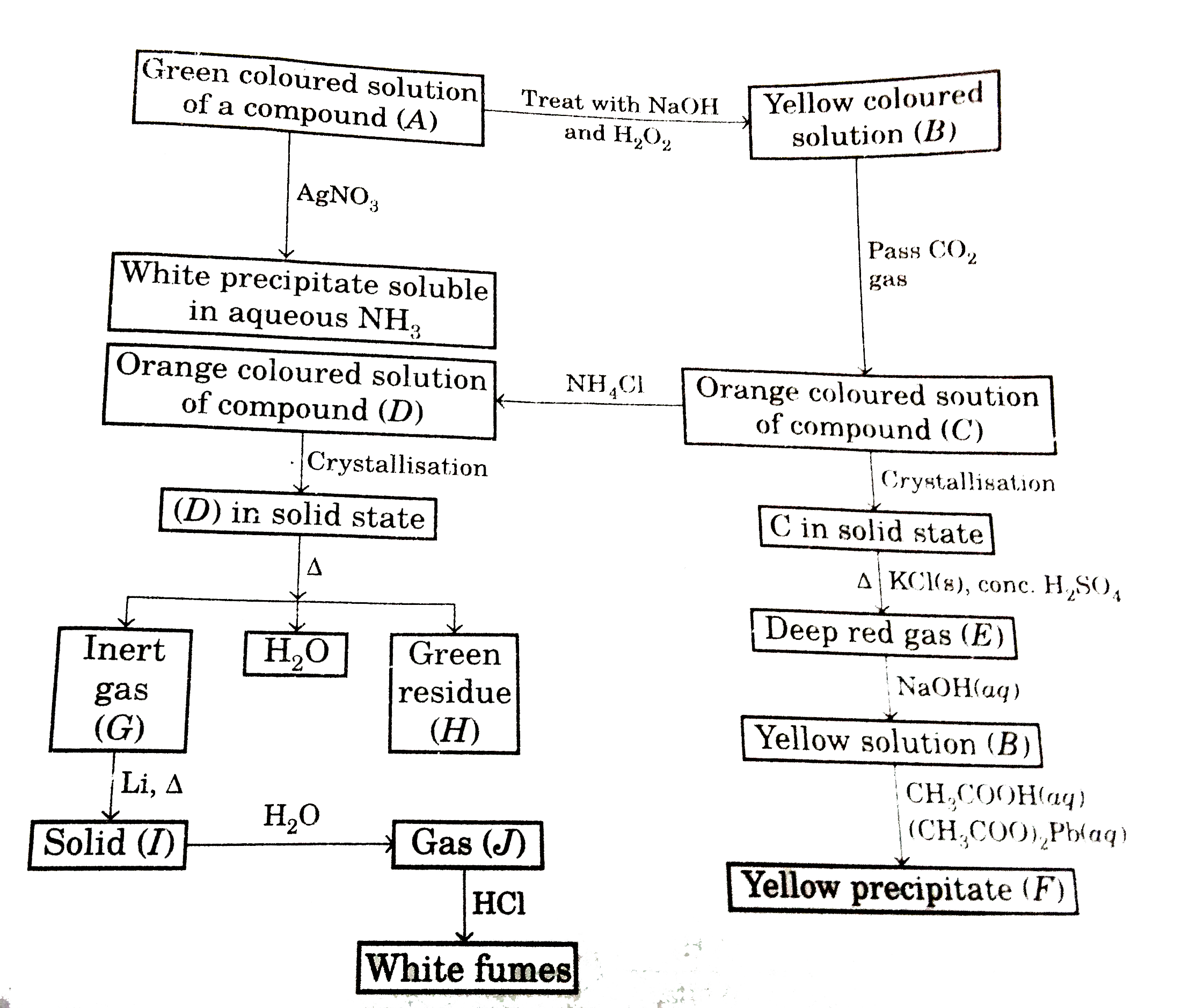Go through the following flow sheet. Based on, it answer the questions given at the end of it.

Select correct statement(s):
A
CO2 makes the solution of (B) acidic and converts (B) into FeCI2(C).
B
CO2 makes the solution of (B) basic and converts it into Na2Cr2O7
C
CO2 makes the solution of (B) acidic and converts it into Na2Cr2O7
D
None of the above is correct
Text Solution
The correct Answer is:C
((A)→CrCI3,,(B)→Na2CrO4,),((C)→Na2Cr2O7,,(D)→(NH4)2Cr2O7),((E)→CrO2CI2,,(F)→PbCrO4,),((G)→N2,,(H)→Cr2O3,),((I)→Li3N,,(J)→NH3)
|
Similar Questions
Knowledge Check
Go through the following flow sheet. Based on, it answer the questions given at the end of it.

Compound (A) is:ACuCI2BFeCI3CFeCI2DCrCI3Go through the following flow sheet. Based on, it answer the questions given at the end of it.

Yellow coloured solution (B) is of:ANa2CrO4 formed by oxidation of Cr3+ by H2O2 in alkaline mediumBFeCI2 formed by reduction of FeCI3 by H2O2CFeCI3 formed by oxidation of FeCI2 by H2O2DCu(OH)2 formed by precipitation of CuCI2 by H2O2/NaOHGo through the following flow sheet. Based on, it answer the questions given at the end of it.

Compounds D,E and F respectively are:ADEF(NH4)2CrO4CrO2CI2PbCrO4BDEF(NH4)2CrO7CrO3PbCrO4CDEF(NH4)2Cr2O7CrO2CI2PbCrO4DDEF(NH4)2CrO4CrO3PbCrO4
Go through th following statements
View SolutionFollowing two graphs are based on the conductometric titration of acid-base reaction. Answer the questions given at the end of it.
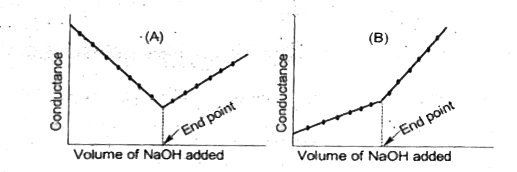
Select correct statement,
View Solution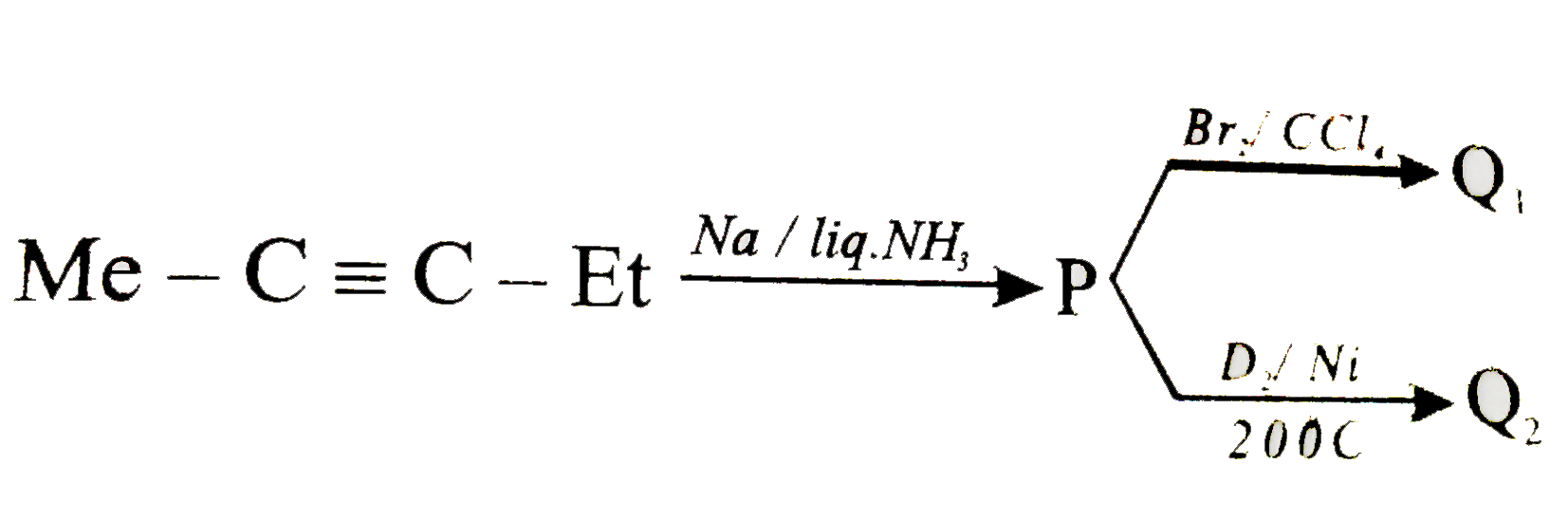
some statement regarding the reaction:
are given below. Select the correct statement(s).
View SolutionThe correct answer to above question is based on
View SolutionRead the given flow chart carefully.

Select the correct statement .
View Solution