FeCI3solution+(NH4)2SNeutral medium−−−−−−−−−→Black ppt.(A) ↓dil.H2SO4White ppt.(B)+Gas(C) while AICI3 solution +(NH4)2S→ White ppt.(D)
What is the formula of black ppt.(A)?
Fe2S3
FeS
2FeS+S
FeS+Fe(OH)3
The correct Answer is:C
Topper's Solved these Questions
- SALT ANALYSISBOOK - GRB PUBLICATIONCHAPTER - SALT ANALYSISEXERCISE - Comprehension 37
1 Video
- SALT ANALYSISBOOK - GRB PUBLICATIONCHAPTER - SALT ANALYSISEXERCISE - Comprehension 38
1 Video
- SALT ANALYSISBOOK - GRB PUBLICATIONCHAPTER - SALT ANALYSISEXERCISE - Comprehension 35
1 Video
- S BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - S BLOCK ELEMENTSEXERCISE - SUBJECTIVE TYPE
9 Videos
- THERMODYNAMICSBOOK - GRB PUBLICATIONCHAPTER - THERMODYNAMICSEXERCISE - All Questions
704 Videos
Similar Questions
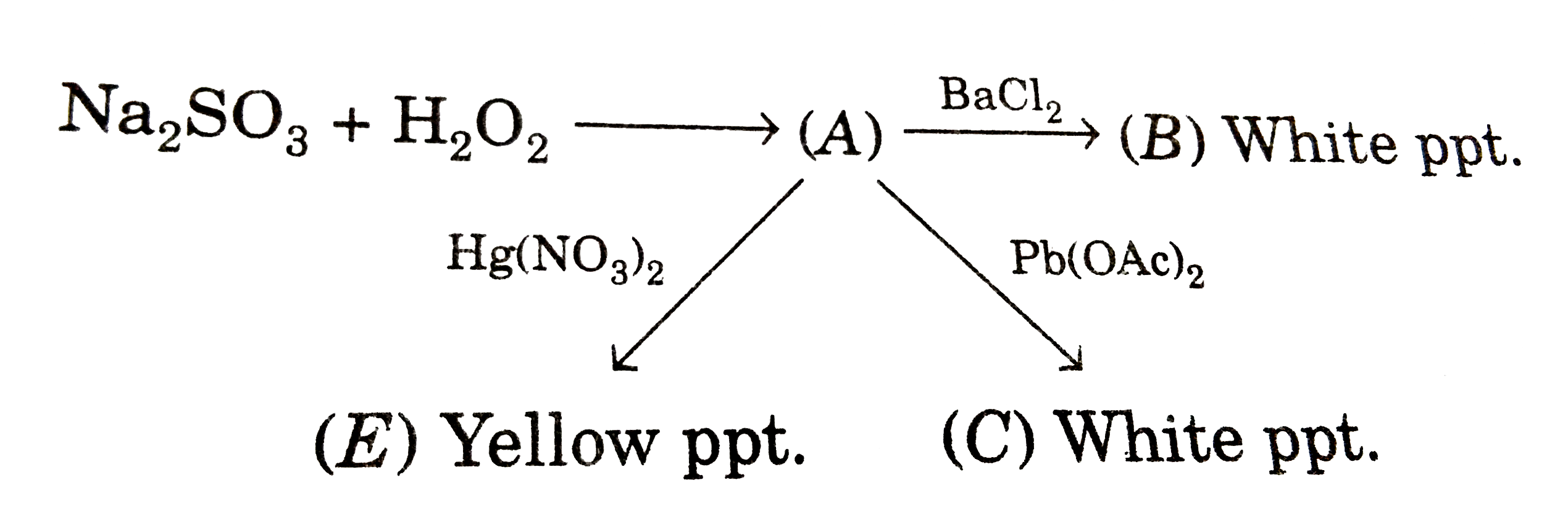
Identify A:
View Solution(A)(Orange solid)Δ−−→(B)(Yellow)+(C)(Green)+(D)(Paramagnetic)
Compound (A) is:
View SolutionWhen a metal rod M is dipped into an aqueous colourless concentrated solution of compound N, the solution turns light blue. Addition of aqueous NaCI to the blue solution gives a white precipitate O. Addition of aqueous NH3 dissolves O and gives an intense blue solution.
The metal rod M is:
View SolutionAmongst the following, the total number of compounds whose aqueous solution turns red litmus paper blue is:
KCNK2SO4(NH4)2C2O4NaCIZN(NO3)2FeCI3K2CO3NH4NO3LiCN
View Solution1 mole of argon is expanded isothermiocally and irreversably ( not against vaccum) from 10L to 100L. Which of the following is incorrect the process ?
View SolutionIdentify (A) and (B) in the following:
KO2+SΔ−−→(A)
(A)+BaCI2→BWhite ppt.
View Solution(A) (colourless solution ) gives white ppt (B) with NaOH solution but ppt dissolves in excess of NaOH forming (C ) (C ) does not give ppt with H_(2)S but on boiling with NH_(4)CI while ppt (B) appears (A) also gives yellow ppt with AgNO_(3) identify (A),(B) and (C )
View Solutionunderset("in basic medium")((D))+(NH_(4))_(2)SO_(4)rarr brown ppt (G) Hence, compound(G) is
View SolutionWhat ppt (B) + NH_(3) rarr Black ppt . (H). Hence, (H) is due to the formula of
View SolutionNH_(3) solution was added to four semple solution in difference test tube and found the following observation about the precipitate. a. White ppt which is solution in oxcess of NH_(3) solution b.On heating which is white in cold but yellow on heating c. The cation present in (b) forms white ppt , with hypo solution which give black ppt on heating d. The cation present in (c ) forms soluble complex with excess of NH_(3) solution White ppts in (a),(b) and (c ) respectively obtained are
View SolutionIdentify (A) to (H) Mineral (A) overset(dil.H_(2)SO_(4))rarr(B) +(C) +(D) (gas) (D) + (CH_(3)COO)_(2)Pb overset(Delta) to black ppt (B) + (C ) overset((D)) to E (black ppt) separrated from (C ) by fitration (E) + underset(dil)(HNO_(3)) overset(Delta) to (F) (blue coloured solution) (F )+ K_(4)[Fe(CN)_(6)] rarr chocolate colourer ppt (C ) + K_(3)[Fe(CN)_(6)] rarr blue (H)
View Solution(White ppt)DNa2CO3←−−−−−−−dil.H2SO4A↓C(White ppt)K2CrO4−−−−−−−−−→(in acetic acid)B(Yellow ppt)
If A is the metallic salt, then the white ppt. of D must be of
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. The ppt white ppt (B) is of
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. White ppt (E) is of
View SolutionFeCI3solution+(NH4)2SNeutral medium−−−−−−−−−→Black ppt.(A) ↓dil.H2SO4White ppt.(B)+Gas(C) while AICI3 solution +(NH4)2S→ White ppt.(D)
White ppt.(D) is :
View Solution

