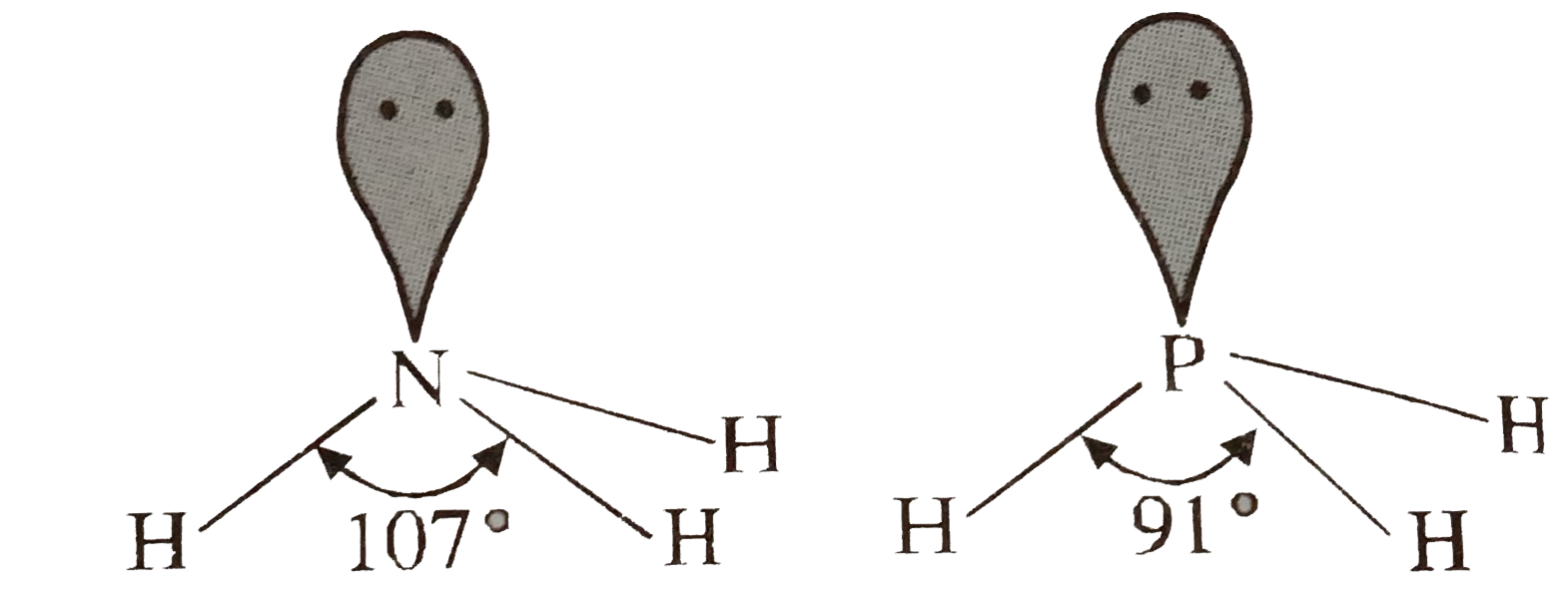
Bond angle in NH3 is molre than in PH3. Explain.
Video Solution
Text Solution
Both NH3 and PH3 are the hybrides of nitrogen and phosphorus which are present in group 15. Nitrogen is more electronegative (3.0) as compared phosphorus (2.1). As a result, the sharet, electron pairs lie closer to the nitrogen atom in NH3 than to the phosphorus atom in PH3. Therefore, the electron density around the nitrogen atom is more than around the phosphous atom. This results in greater repulsion in the electron pairs around nitrogen atom than around phosphorus atom. As a result, bond angle in NH3 is more than in PH3.
|
Similar Questions
Knowledge Check
Bond angle in PH3 is
AMuch less than NH3BMuch less than PF3CSlightly more than NH3DMuch more than PF3Bond angle in PH3 is
AMuch less than NH3BMuch less than PF3CMore than NH3DMore than PF3Bond angle in NH3 molecule is
A109∘28∘B107∘18∘C104∘9∘D90∘
