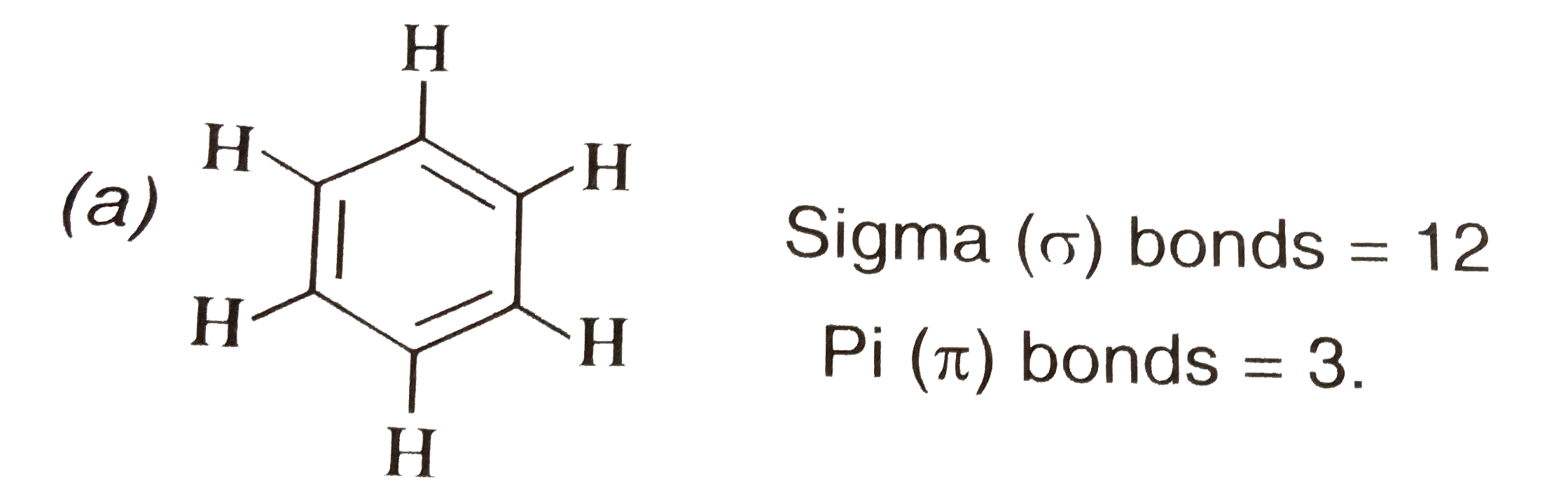In the resonating structures of of benzene, the number of σandπ bonds are:
A
3πand12σ
B
3σand12π
C
6πand6σ
D
12πand12σ
Video Solution
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
In the resonating structure of aniline, the number of σ and π bonds are respectively
A12,σ,3πB14σ,3πC6σ,6πD3σ,3π.Number of σ and π bonds present in :
A10σ,3πB10σ,2πC9σ,2πD8σ,3πThe number of sigma and pi bonds in benzene are
A6σ and 3π bondsB12σ and 3π bondsC9σ and 3π bondsD6σ and 6π bonds
The number of σ bonds, π bonds and lone pair
View SolutionHow resonating structures are possible for benzene?
View SolutionThe number of sp2−s sigma bonds in benzene are
View SolutionNumber of resonating structures for Dewar's benzene will be
View SolutionThe number of σ and π-bonds in but-1-en-3-yne are
View Solution