when benzyl chloride is boiled with aqueous KOH solution and the solution is acidified by a few drops of dilute HNO3 followed by AgNO3 solution, a white precipitate is formed. However, no such precipitate is noticed when the reaction is carried with chlorobenzene. explain.
In case of C6H5CH2Cl, the benzyl carbocation (C6H5⊕CH2) formed by releasing Cl− ion is resonance stabilised. Therefore, the C−Cl bond can cleave to form KCl. However, the phenyl carbocation (C6⊕H5) expected to be formed in case of chlorobenzene does not show any resonance. therefore, C−Cl bond does not cleave in case of chlorobenzene and no white precipitate is formed in this case.
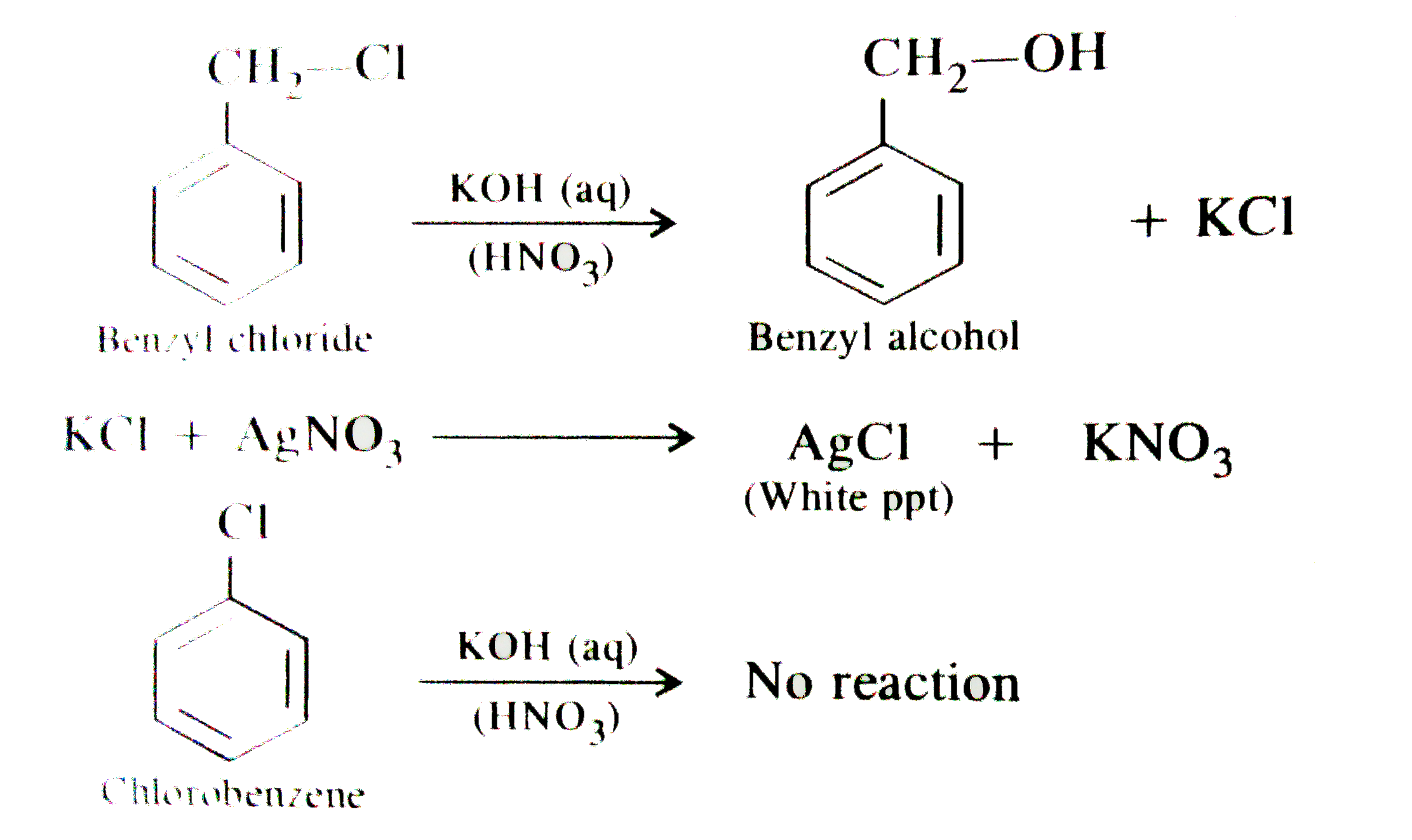
Similar Questions
Chlorobenzene and benzyl chloride can be distinguished by boiling with aqueous KOH followed by acidification with dil HNO3 and subsequent treatment with AgNO3 solution.
View SolutionWhen aqueous KCN is added to a solution of copper (i), a white precipitate, soluble in a excess of KCN is formed. No precipitate is formed when H2S is passed into it Explain.
View Solution
Knowledge Check
AgNO3 solution will not give white precipitate with
A[Co(NH3)3Cl3]B[Co(NH3)4Cl2]ClC[Co(NH3)5Cl]Cl2D[Co(NH3)6]Cl3In Carius tube, the compound ClCH2COOH was heated with fuming HNO3 and AgNO3. After filtration and washing, a white precipitate was formed. The precipitate is of
AAgClBAgNO3CAg2SO4DCH2ClCOOAgIn Carius tube, the compound ClCH2COOAg was heated with fuming HNO3&AgNO3. After filtration and washing, a white precipitate was formed. The precipitate is
AAg2SO4BAgNO3CAgClDClCH2COOAg
The solution of BiCl3 in dil. HCl when diluted with water , white precipitate is formed which is
View SolutionWhen aqueous solution of potassium fluoride is added to the blue coloured aqueous CuSO4 solution , a green precipitate is formed . This observation can be explained as follows .
View SolutionAqueous solution of a salt when treated with AgNO3 solution gives a white precipitate , white dissolves in NH4OH. Radical present in the salt is
View SolutionOn mixing aqueous solutions of silver nitrate and sodium chloride, a white precipitate. is obtained. This reaction can be categorized as reaction.
View SolutionOn mixing aqueous solutions of silver nitrate and sodium chloride, a white precipitate. is obtained. This reaction can be categorized as reaction.
View Solution

