Which of the following ions is more stable ?
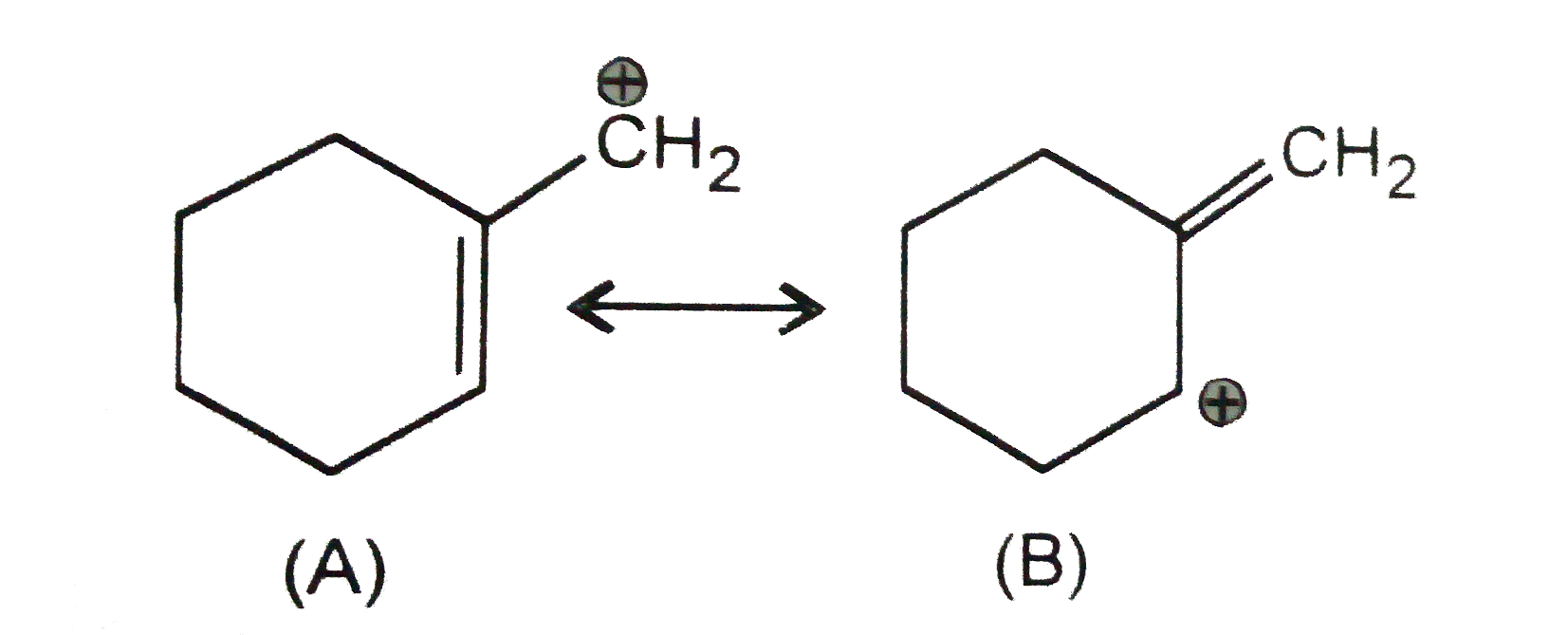
Structure [A] is more stable as compound to structure (B). Actually, an andocyclic double bond (present within the ring) is present in structure [A]. On the other hand, an exocyclic double bond (present outside the ring) is present in structure [B]. As a result, structure [A] is more stable as compared to structure [B].
Topper's Solved these Questions
Similar Questions
Chromatography is a recent and most effective technique which was used by Tsweff, a botanist for the separation of coloured substances into individual constutients. The name Chromatography means colour writing (In Greek , Chrome = colour, graphy = writing). However, the name as such has lost its significance. The technique can be used to separate constituents from any mixture whether coloured or colourless.
(i) What is the basic of Chromatographic separation ?
(ii) What is the value associated with it ?
View Solutionनिम्न में से कौन-से आयन अधिक स्थायी है?
View SolutionWhich of the following ions is more stable in aqueous solutions
View SolutionWhich carbonium ion is more stable in the given cations
View SolutionWhich of the following ions is more stable? Use resonance to explain your answer

View SolutionWhich of the following ions is more stable? Use resonance to explain your answer

View SolutionWhich of the atoms and ions is more stable and why?
View SolutionBetween Fe^(2+) ion and Fe^(3+) ion which is more stable ?
View Solution
