Why does pure HCN fail to react with aldehydes and ketones ?
Video Solution
Text Solution
HCN is mainly a covalent compound and can not provides CN− ion readily for the nucleophilic attack on the carbonyl compound. The reaction is carried in the presence of base which helps in the ionisation of the acid to liberate CN− ion.
HCN+OH−→H2O+CN−
|
Similar Questions
Give suitable explanation for the following:
(i) Chloral hydrate is a geminaldiol but still stable. How?
(ii) Out of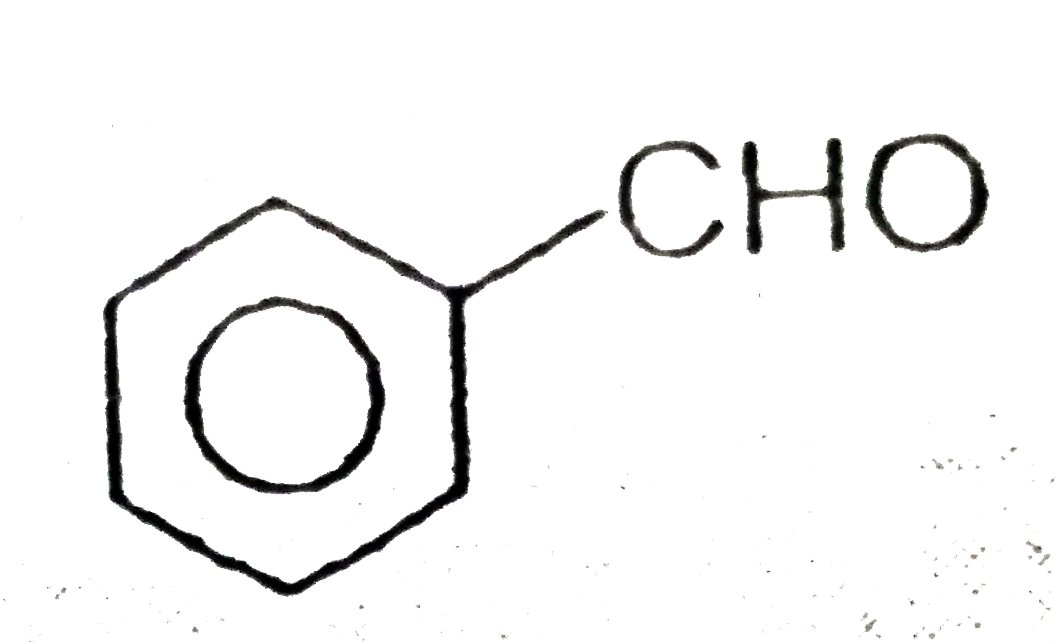 and CH3CH2CHO which is more reactive nucleophilic addition?
and CH3CH2CHO which is more reactive nucleophilic addition?
(iii) Why does pure HCN fail to reac with aldehydes and ketones?
(iv) Hydrazones of aldehydes and ketones are not prepared in strongly acidic mediu. Why?
(v) Sodium bisulphite is used to purify aldehydes and ketones. Explain.
View SolutionReactions of aldehyde and ketones
View Solution
Knowledge Check
In aldehydes and ketones
ACarbonyl carbon is nucleophilic and carbonyl oxygen is electrophilicBCarbonyl carbon, is slectrophilic and carbonyl oxygen is nucleophilicCBoth are electrophilicDBoth are nucleophilicAldehydes and ketones are
AChain isomersBFunctional isomersCposition isomersDOptical isomersAldehyde and ketones are
AHomologousBIsomersCGeometricalDConformational
