Why is pKa of F−CH2COOH lower than that of Cl−CH2COOH?
Fluorine (-F) has a stronger -I effect as compared to chlorine (-Cl). Therefore, release of H+ ion from ∝-fluoroacetic acid is easier than from β-chloroacetic acid.
Similar Questions
(a) Write the chemical equation for the reaction involved in Cannizzaro reaction.
(b) Draw the structure of the semicarbazone of ethanal.
(c ) Why pKa of F−CH2-COOH is lower than that of Cl−CH2−COOH?
(d) Write the product in the following reaction :
CH3−CH=CH−CH2CN(i)DIBAl−H−−−−−−−−→(ii)H2O
(e) How can you distinguish between propanal and propanone?
View Solution(A) Write the chemical equation for the reaction involved in Cannizzaro reaction.
(b) Draw the structure of the semicarbazone of ethanal.
(c ) Why pKa of F−CH2−COOH is lower than that of Cl−CH2−COOH ?
(d) Write the product in the following reaction :
CH3−CH=CH−CH2CN(i)DIBAL−H−−−−−−−−−−−→(ii)H2O
(e ) How can you distinguish between propanal and propanone ?
View Solution
Knowledge Check
The reaction,
CH3COOH+Cl2P−−→ClCH2COOH+HCl
is calledAHell-Volhard-Zelinsky reactionBFischer reactionCRosenmund reactionDFriedel Craft's reactionArrange the following carboxylic acid in their decreasing acidity
(1) COOH|COOH Oxalic acid
(2) HOOC−CH2−COOH Molonic acid
(3) CH2−COOH|CH2−COOH Succinic acidA3>2>1B1>2>3C2>3>1D2>1>3The reaction,
CH3COOH+Cl2P−−→ClCH2COOH+HCl
is calledAHell-Volhard-Zelinsky reactionBBirch reactionCRosenmund reactionDHunsdiecker reaction
Why is CH2=CH−COOH a stronger acid than CH3CH2COOH?
View SolutionGive reasons :
(a) Propanone is less reactive than ethanal towards nucleophilic addition reactions.
(b) O2N−CH2−COOH has lower pKa value than CH3COOH
( c) (CH3)2CH−CHO undergoes aldol condensation whereas (CH3)3C−CHO does not.
View SolutionMe3CCH2COOH is more acidic than Me3SiCH2COOH.
View SolutionWhich is the stronger acid in the following pair?
CH3−CH2−COOHandNC−CH2−COOH
View Solution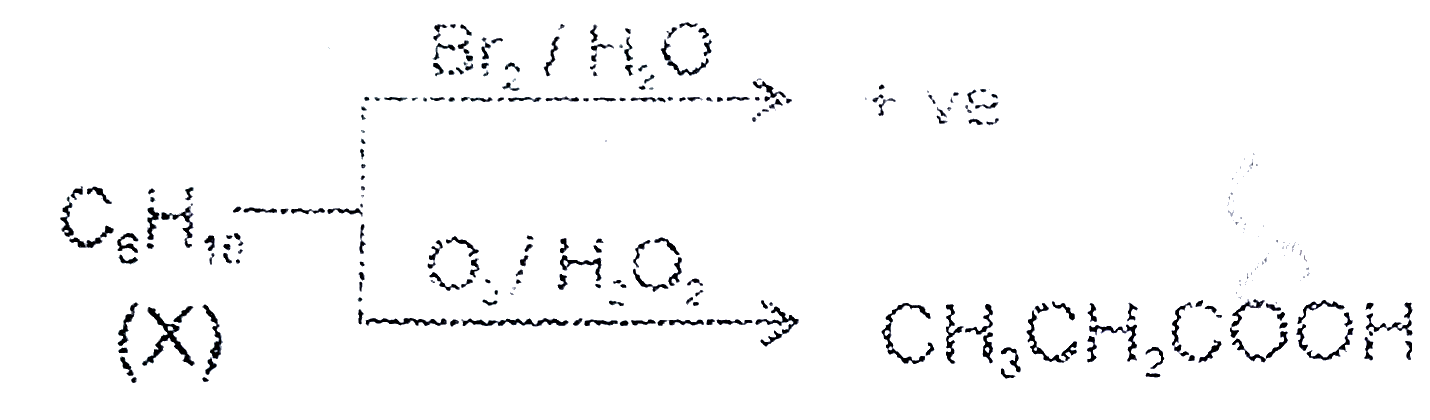 , CH3CH2COOH
, CH3CH2COOH
Identify X:
View Solution
