Consider the following reaction
C4H8O2Ester(x)(i)CH3MgBr(Excess)−−−−−−−−−−−−−−→(ii)H3O+C4H10OAlcohol(Y) Alcohol (Y) reacts fastest with Lucas reagent. Therefore (X) and (Y) are
CH3COOC2H5 and (CH3)3COH
HCOOC3H7 and (CH3)2CHOH
C2H5COOCH3 and (C2H5)3COH
HCOOC3H7 and (CH3)3COH.
The correct Answer is:A
(a) The alcohol is a tertiary alcohol (CH3)3COH since it reacts fastest with Lucas reagent. CH3COOC2H5 will form this alcohol. Ester HCOOC3H7(d) will not form teritary alcohol. It will give secondary alcohol.
Similar Questions
C3H6Cl2A(i) KCN−−−−−−→(ii) H3O+(iii) ΔCH3CH| CH3COOH. Hence, A is
View SolutionIdentify (A) in the following reaction sequence underset("iodoform test")underset("Gives Positive")(A)underset(underset("(iii) Conc. "H_(2)SO_(4)//Delta)("(ii) "H^(+),H_(2)O))overset("(i) "CH_(3)MgBr)rarr(B)overset(O_(3)//Zn,H_(2)O)rarr
View SolutionConsider the following reaction :
C6H5−CH3∣C∣H(R)−Cl+C2H5⊕O→C2H5O−CH3∣C∣H100%(S)form−C5H5
In the given
C6H5−⋅⋅S⋅⋅−CH2−H∣C∣CH3−Br+C2H5⊕O→(P) the product is :
View SolutionC8H12O2LiAlH4−−−→H2OB(two mol per mol of A)PCC−−→(C)CH3MgBr−−−−−→H3O+(D)H2CrO4−−−−−−−−→Acetone/water
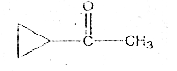
(A) Identify A to D.
View SolutionCH3C≡CCH3(i)X−−−−−−−−−→(ii)H2O/ZnH3C−C∣∣O−C∣∣O−CH3
In the above reaction X is
View SolutionIn the following reaction sequence, CH_(3) - underset((X))underset(OH)underset(|)(C)H-CH_(3) overset([O])(to) Y overset(CH_(3)MgBr)underset(H^(+)// H_(2)O)(to) Z Z is
View Solutionनिम्न चार संरचनाओं के लिए
C2H5−CH3∣CH−C3H7(I) CH3−O∣∣C−CH3∣CH−C2H5(II)
H−H∣C⊕∣H(III) C2H5−CH3∣CH−C2H5(IV)
सत्य कथन है
View SolutionIdentify (A) in the following reaction sequence
AGives Positiveiodoform test(i) CH3MgBr−−−−−−−−−−−→(ii) H+,H2O(iii) Conc. H2SO4/Δ(B)Oy/Zn,H2O−−−−−−−→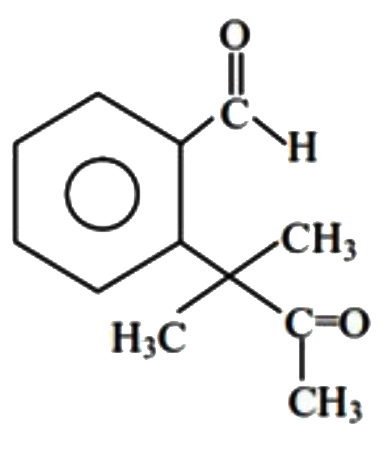
View Solution

