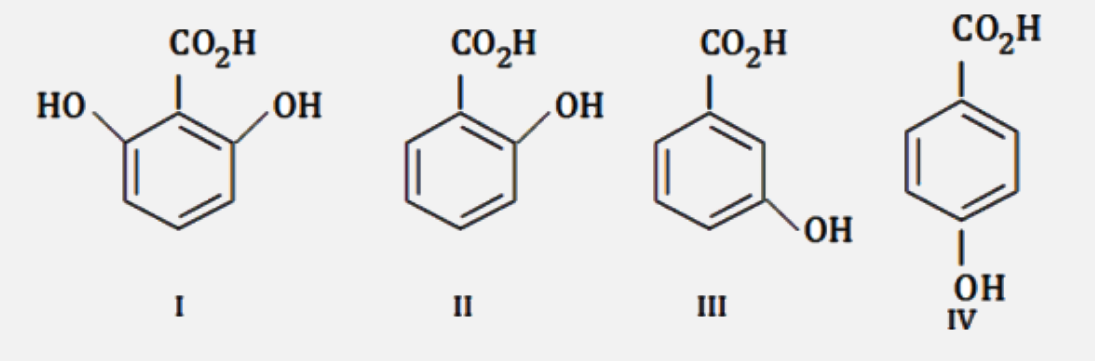
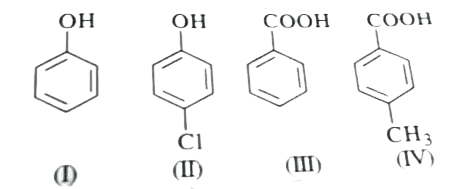
The correct order of acidity for the following compounds is
A
I>II>III>IV
B
III>I>II>IV
C
III>IV>II>I
D
I>III>IV>II
Text Solution
The correct Answer is:B, C
(a) It is the correct order. It is explained on the basis of ortho effect and hydrogen bonding. Ortho effect is noticed in both (I) and (II). But acid (I) is stronger because intermolecular hydrogen bonding in the conjugate base of (I) is more than that of (II) since there are two OH groups present. Acid (II) is stranger acid than acid (I).
|
Similar Questions
Knowledge Check
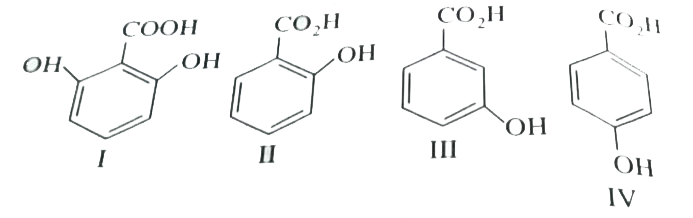
The correct order of acidity for the following compounds is
 AI>II>III>IVBIII>I>II>IVCIII>IV>II>IDI>III>IV>II
AI>II>III>IVBIII>I>II>IVCIII>IV>II>IDI>III>IV>IIThe correct order of acidity for the following compound is
ABenzoic acid > phenol > p-nitrobenzoic acid > m-nitrobenzoic acid.BPhenol > p-nitrobenzoic acid > m-nitrobenzoic acid > benzoic acidCp-nitrobenzoic acid > m-nitrobenzoic acid > benzoic acid > phenol.Dm-nitrobenzoic acid > p-nitrobenzoic acid > benzoic acid > phenol.the correct order of acidity of the following compounds is
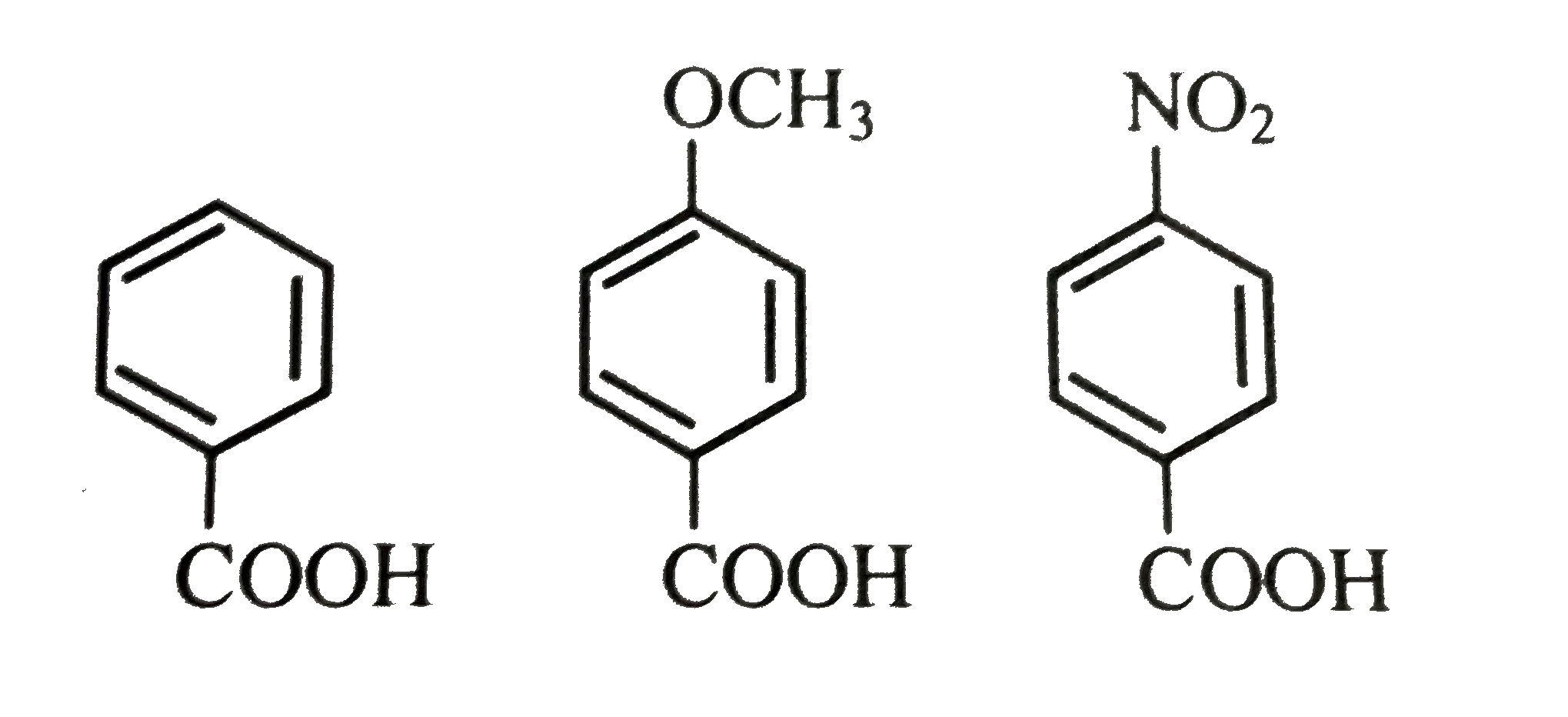 A1>2>3B1>3>2C3>1>2D3>2>1
A1>2>3B1>3>2C3>1>2D3>2>1
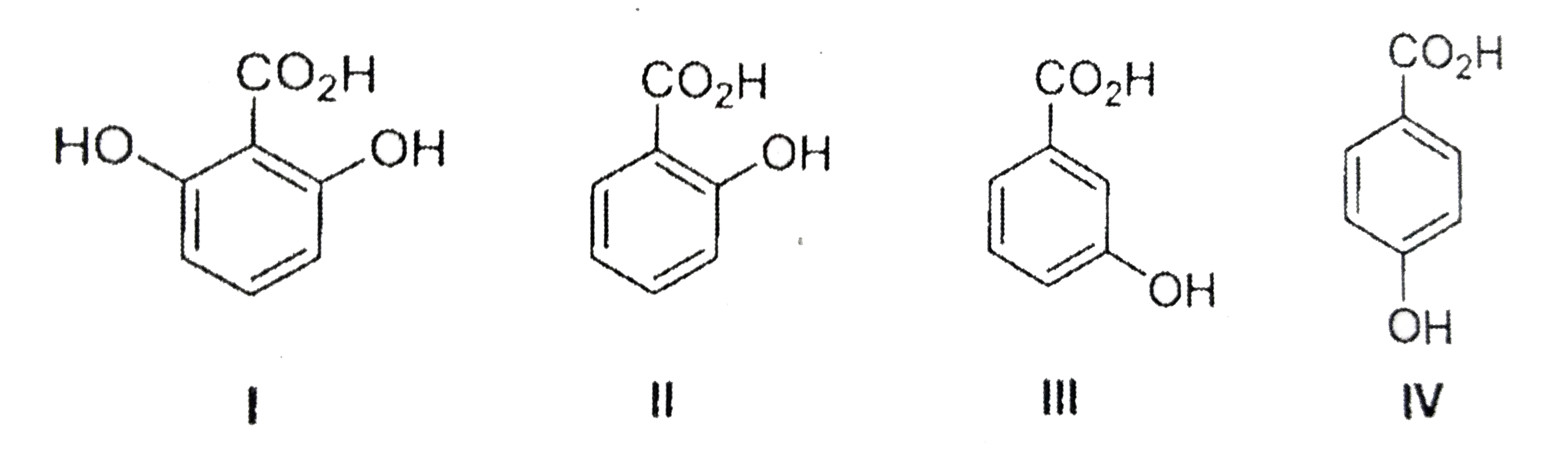
The correct order of acidity for the following is
View SolutionThe correct order of acidity of the following compounds is:
(I)CH3COOH (II)ClCH2COOH (III)O2NCH2COOH (IV)HOCH2COOH
View SolutionThe correct order of acidity of the following is
View SolutionThe correct order of acidity of the following is :
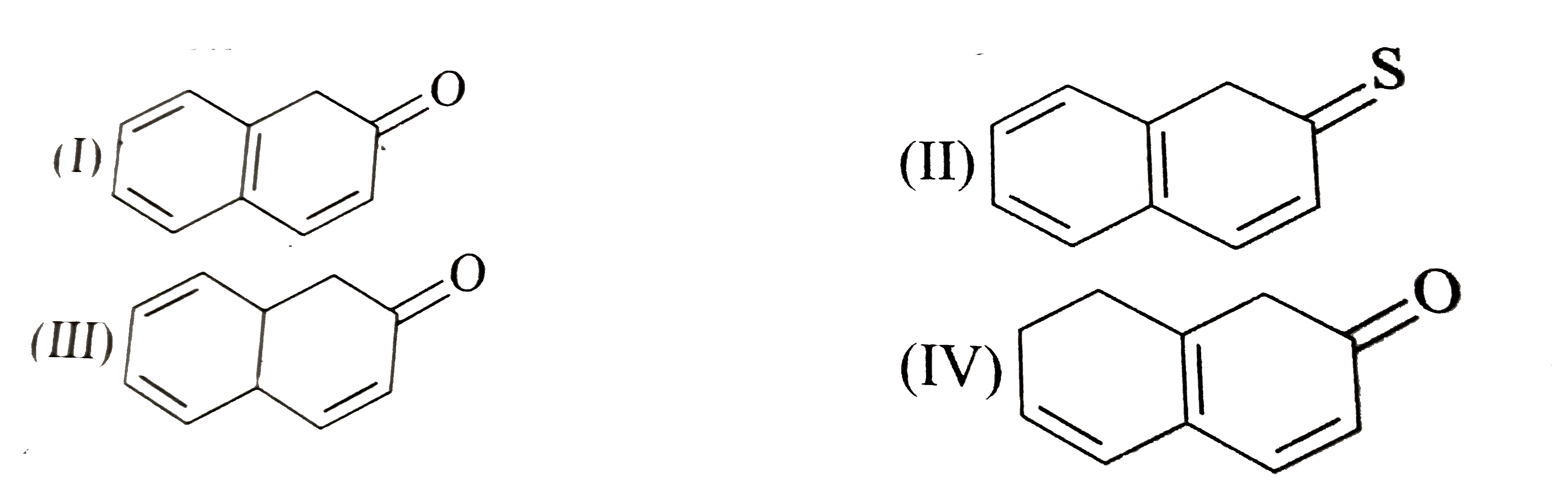
View SolutionThe correct order of basicities of the following compounds is

View Solution