When But-3-en-2-ol reacts with aqueous solution of HBr, the product is a mixture of 3-Bromobut-1-ene and 1-Bromobut-2-ene. Explain.
Video Solution
Text Solution
A mixture of carbocation is formed as a result of initial attack of electrophile (H+ ion) of the acid. The nucleophile (Br− ion) reacts with both of them to form a mixture of products.
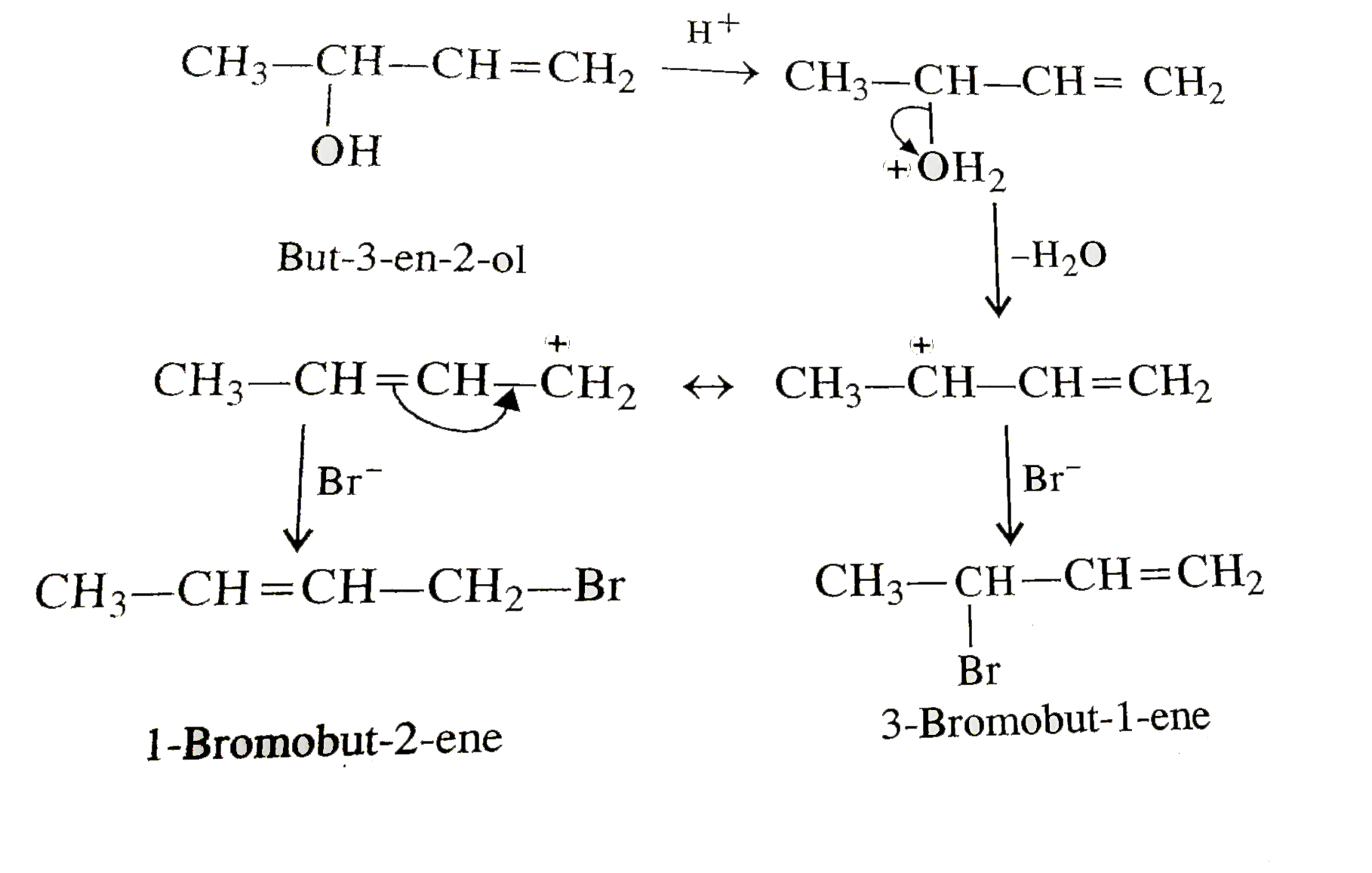
|
Similar Questions
Knowledge Check
When but −3−en−2−ol reacts with aqHBr , we get
ACH3CH∣BrCH=CH2BCH3CH=CHCH2BrCboth (1) and (2)DH2C=CHCH=CH2But-1-ene and But-2-ene are
AGeometrical isomersBChain isomersCPosition isomersDOptical isomersWhen but −3−en−2−ol reacts with aqHBr , we get
A3 - bromobut -1- eneB1 - bromobut -2- eneCa mixture of both A and BD2 - bromobut -2- ene
When but −3−en−2−ol reacts with aqHBr , we get
View SolutionThe structure of 3-bromoprop-1 -ene is
View SolutionBut-1-ene and but-2-ene can be distinguished by
View SolutionWhen 3-ethylpent-2-ene reacted with bromine water the major product as (IUPAC name):
View SolutionThe product of the reaction
But-2-ene+NBr→?
View Solution

