Explain why si orthonitrophenol ?
Text Solution
Nitro (NO2) group is an electron withdrawing group while methoxy (OCH3) group is electron releasing in nature. The release of H+ ion is therefore, easier from o-nitrophenol while it is quite difficult from o-methoxyphenol. Apart form that, o-nitrophenoxide ion is stabilised due to resonance (for the resonating structures refer to Q. No. 8,. On the contary, o-methoxyphenoxide is destabilised since the electron density on the negatively charged oxygen tends to increase due to the electron releasing tendency of the methoxy (OCH3) group.

In the light of the above disscussion, we may conclude that o-nitrophenol is a stronger acid (pKa=7.23) than o-methoxyphenol (pKa=9.98).
|
Similar Questions
Explain why orthonitrophenol is more acidic than orthomethoxyphenol?
View SolutionGive teh reason for the following :
Orthonitrophenol is more acidic than orthomethoxy phenol.
View SolutionExplain why the system are not aromatic?

View SolutionExplain why the system are not aromatic?
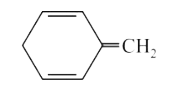
View SolutionExplain why the system are not aromatic?

View SolutionExplain why the system are not aromatic?

View SolutionExplain why the system are not aromatic?
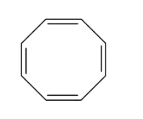
View SolutionExplain why the system are not aromatic?

View SolutionThe SI unit of work or energy is joule. Explain why the work and energy have same SI unit.
View SolutionExplain why there is no waste in a forest.
View Solution

