Hydration of 3-phenylbut-1-ene in dilute H3SO4 forms 2-pheylbutan -2-ol and not 3-phenylbutan-2-ol. Why ?
Video Solution
Text Solution
The secondary carbocation initially formed as a result of hydration changes to a more stable tertiary carbocation by H− ion shift. Therefore, the final product is different from the expected product.
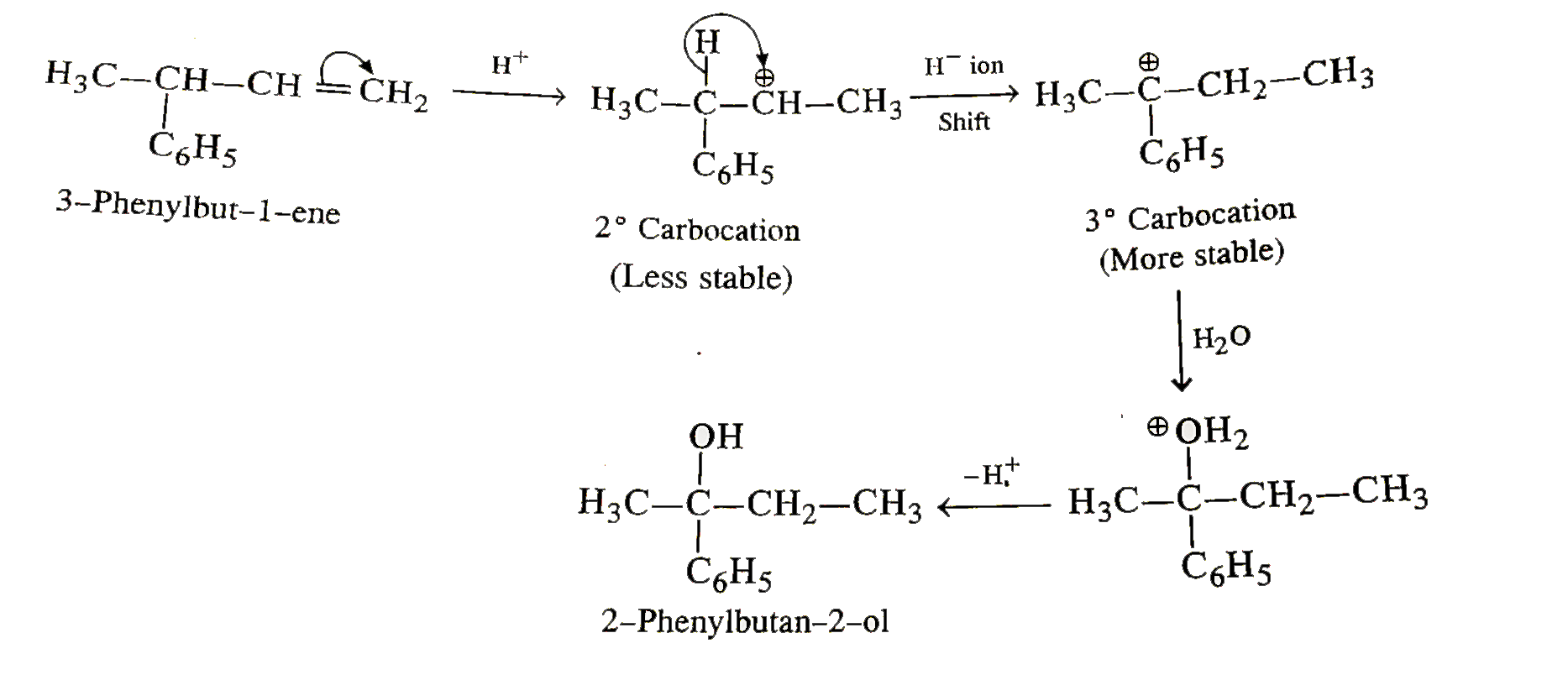
|
Similar Questions
Knowledge Check
Hydration of 3-phenylbut-1-ene in dil. H2SO4 will give mainly
A3-Phenylbutan-1-olB3-Phenylbutan-2-olC2-Phenylbutan-2-olD2-Phenylbutan-2-olHydration of 3-phenylbutan-1-ene in dil. H2SO4 will give mainly :
A3-Phenylbutan-2-olB3-Phenylbutan-2-olC2-Phenylbutan-2-olD2-Phenylbutan -1-olDehydration of butan −2−ol with conc. H2SO4 gives preferred product.
Abut−1−eneBbut −2− eneCpropeneDethane
3-methylbut-3-en-1-ol
View Solution3-bromo-2-mathylpentan-2-ol
View SolutionWhich isomerism id present in Pentan-1-ol and 3-Methylbutan-2-ol ?
View SolutionButan-1-ol reacts with cone. H2SO4 to give but-2-ene.
View SolutionArrange the following in order of decreasing boiling points
(i) Pentan-1-ol (ii) 2-Methylbutan-2-ol, (iii) 3-Methylbutan-2-ol.
View Solution
