How will you distinguish between allyl alcohom and n-propyl alcohol ?
Both are primary alcohols. However, allyl alcohol will give white turbidity with Lucas reagent immediately, but n-propyl alcohol does not react at room temperature. Actually in allyl alcohol, the allyl carbocation left after the release of OH− ion is resonance stabilised which means that C−OH bond can be very easily cleaved by HCl. But the C−OH bond cleavage is not so easy in n-propyl carbocation is not resonance stabilised.
CH2=CH−CH2−OHH+(Acid)−−−−−−−→CH2=CH−CH2−+OH2−H2O−−−→[CH2=CH−⊕CH2↔⊕CH2−CH=CH2]
Similar Questions
How will you distinguish between benzyl alcohol and phenol ?
View SolutionComplete the following:
i. Give the oxidation product of
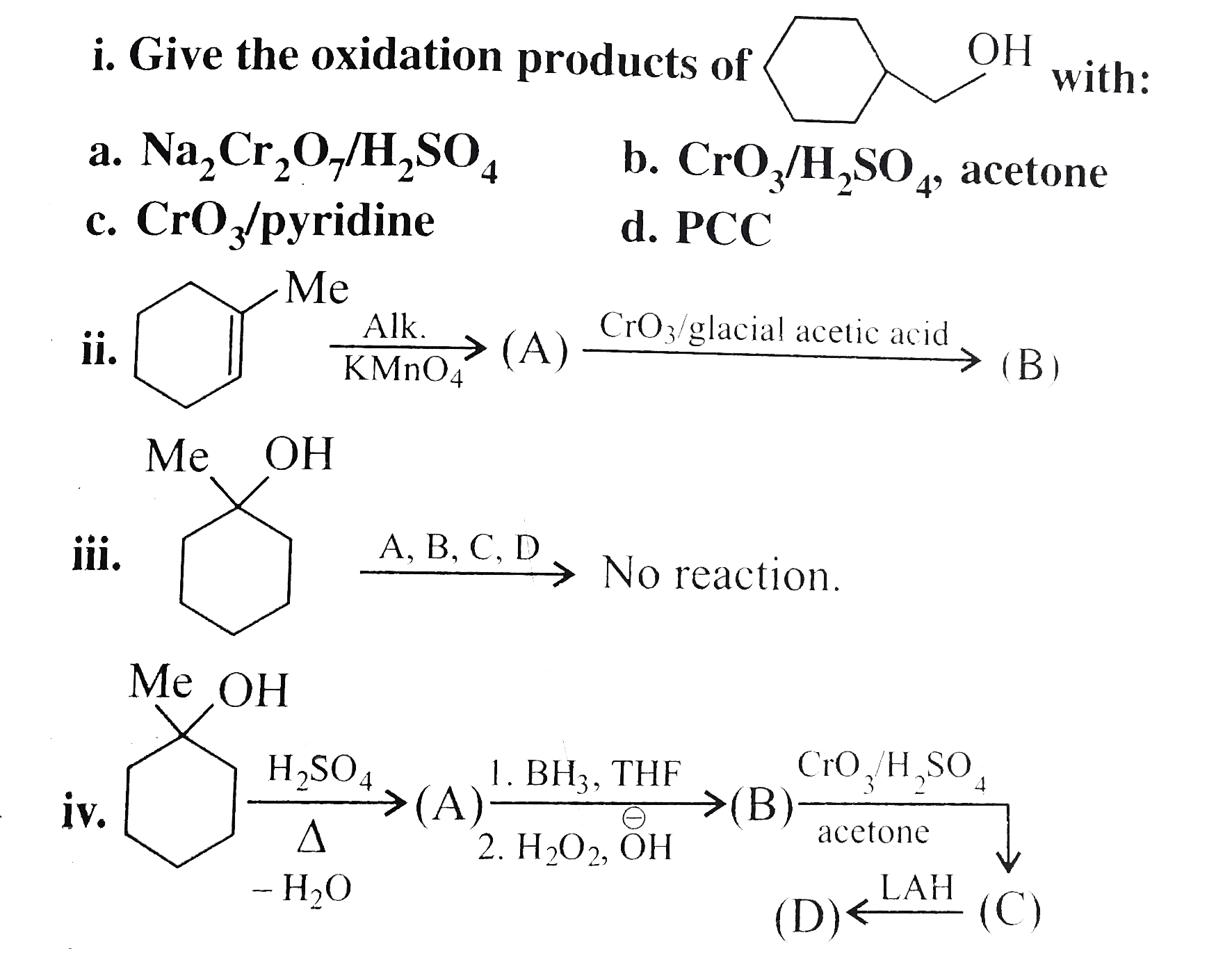
v. Distinguish between allyl alcohol (A) and n-propyl alcohol (B).
vi. Complete the following:
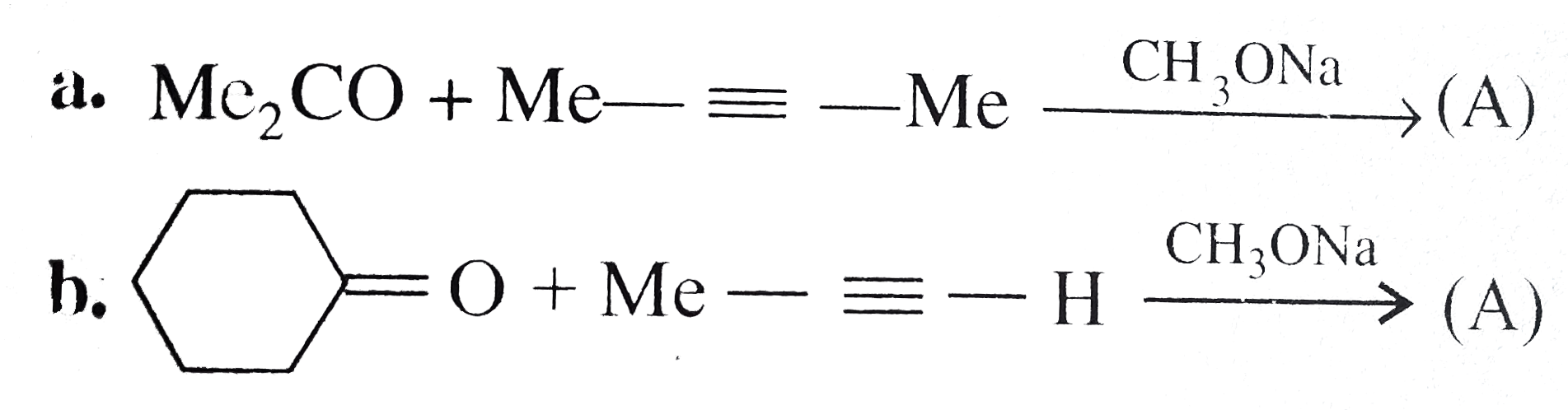
c. CH3CHO+HC≡CDCH3ONa−−−−−→(A)
d.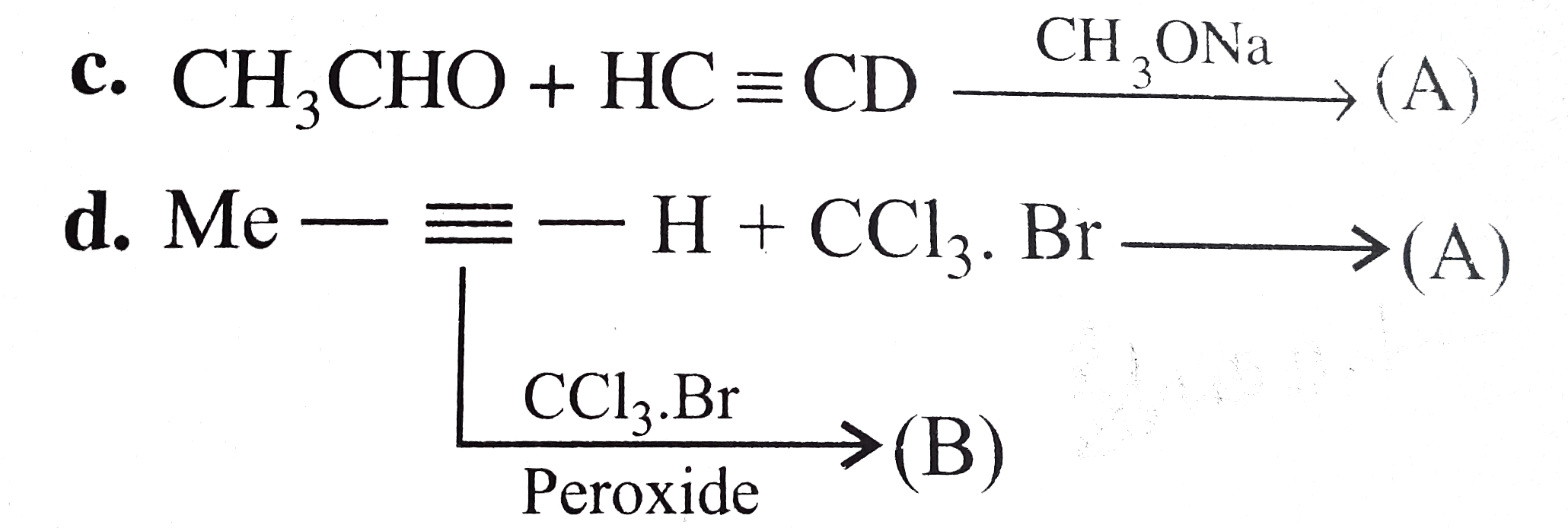
vii. Write the stereochmical products of the following:
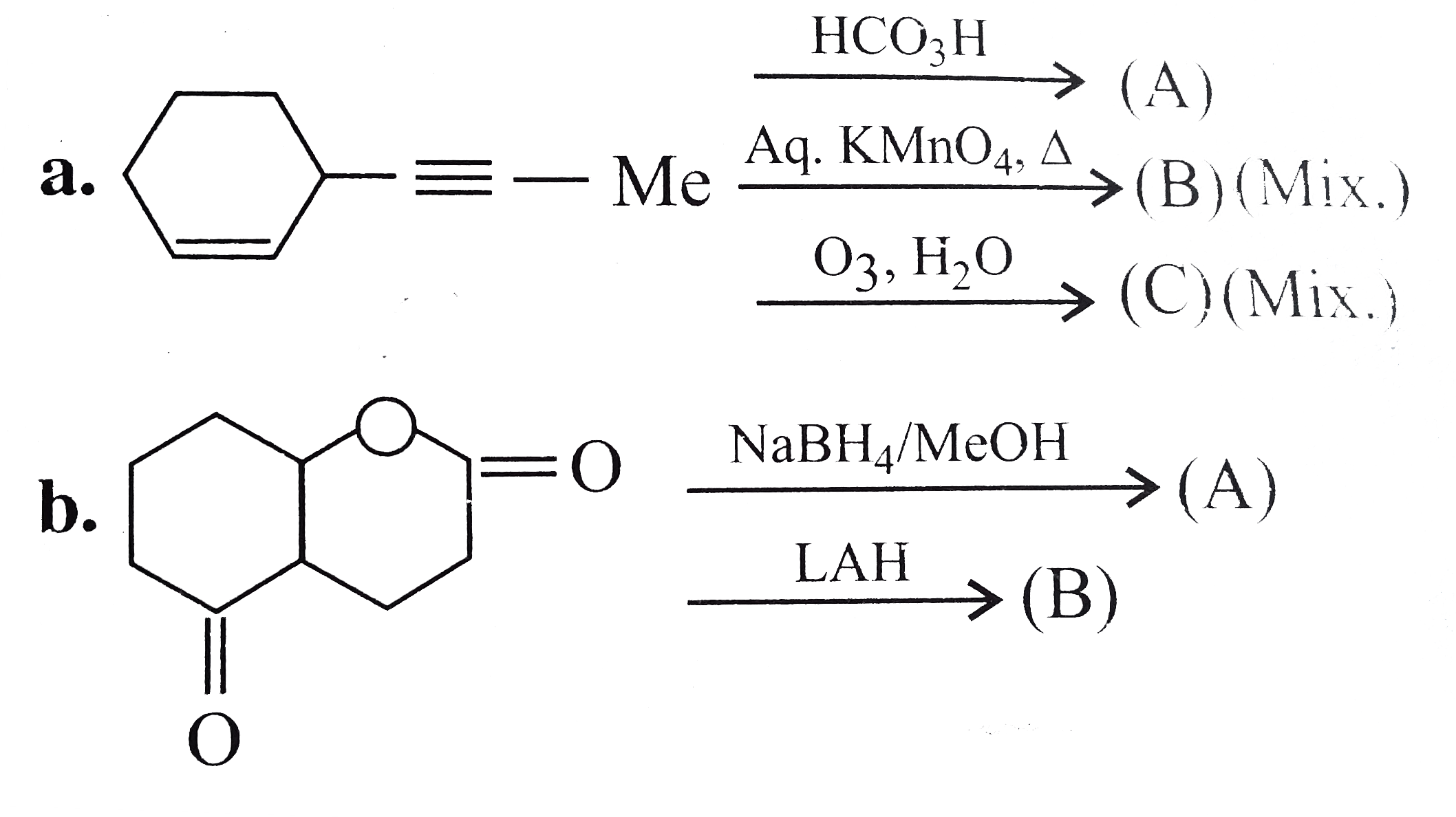
View Solution
Knowledge Check
How would you distinguish between
Areversible and irreversible reactionsBphysical and chemical equilibriaCstatic and dynamic equilibriaDvelocity constant and equilibrium constant
How will you distinguish between propanal and propanone?
View SolutionGive chemical tests to distinguish between
(i) Isopropyl alcohol and n-propyl alcohol
(ii) Phenol and alcohol
(iii) Methyl ethanoate and ethyl ethanoate.
View SolutionGive chemical tests to distinguish between :
(i) Isopropyl alcohol and n - propyl alcohol
(ii) Phenol and alcohol
(iii) Methyl ethanoate and ethyl ethanoate.
View SolutionHow will you distinguish between aldehydes and ketones?
View SolutionHow will you distinguish between ethyl chloride and vinyl chloride?
View SolutionHow will you distinguish between ethyl chloride and vinyl chloride?
View SolutionHow would you distinguish experimentally between an alcohol and a carboxylic acid?
View Solution
