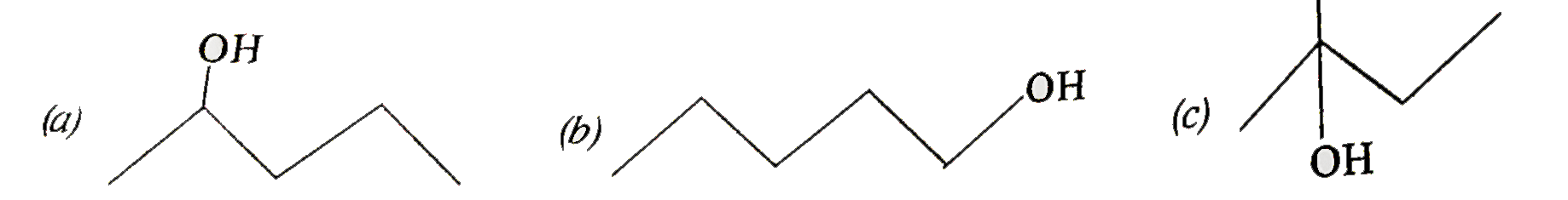
Predict in which of the following cases, the reaction with Lucas reagent will be immediate, slow or will not take place at all room temperature ?
Video Solution
Text Solution
(a) Structural formula represents a secondary alcohol. Therefore, it will give white turbidity with Lucas reagent after some time i.e. the reaction is slow.
(b) Structural formula is of a primary alcohol which gives white turbidity with Lucas reagent only upon heating.
(c) Structural formula is of a tertiary alcohol and it will give white turbidity with Lucas reagent immediately.
|
Similar Questions
Knowledge Check
Which of the following compound does not react with Lucas reagent ?
A(CH3)3C−CHOB(CH3)3C−OHC(CH3)2CH−OHDCH3−OHWhich of the following will react fastest with Lucas reagent?
AButan-1-olBButan-2-olC2-Methylpropan-2-olD2-MethylpropanWhich of the following reacts first with Lucas reagent :
AC3H7OHB(CH3)2CHOHC(CH3)3COHDC6H5OH
In which of the following cases is the reaction spontaneous at all temperatures?
View SolutionIn which of the following cases is the reaction spontaneous at all temperatures?
View SolutionWhich of the following compounds reacts fastest with Lucas reagent?
View SolutionWhich of the following compounds reacts immediately with Lucas reagent ?
View SolutionWhich of the following reactions will not take place ?
View Solution

