The representation of the ground state electronic configuration of He by box diagram as 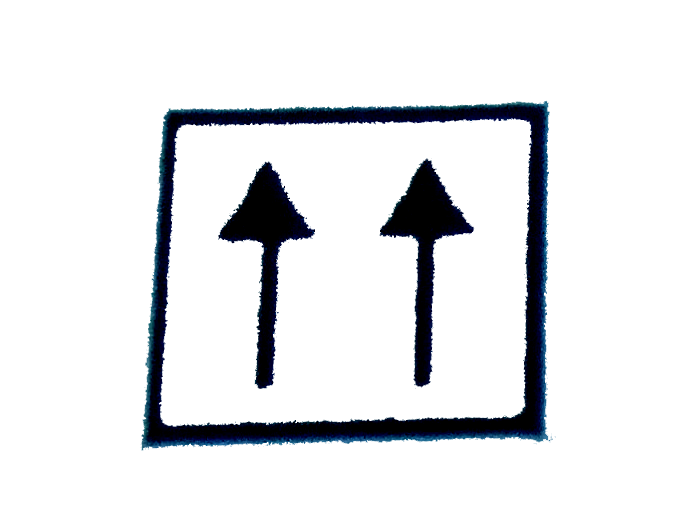 is wrong because it violates
is wrong because it violates
A
Heisenberg's uncertainity principle
B
Bohr's quantization theory of Angular Momentum
C
Pauli's exclusion principle
D
Hund's rule
Video Solution
Text Solution
The correct Answer is:C
According to Pauli's exclusion principle, two electrons in the same orbital should have opposite spins.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Ground state electronic configuration of Cr atom is :
A[Ar]3d44s2B[Ar]3d54s1C[Ar]3d(6)4s0D[Ar]3d54s2The ground state electronic configuration of CO molecule
A1σ22σ21π43σ2B1σ22σ23σ21π22π2C1σ22σ21π23σ22π2D1σ21π42σ23σ2The ground state electronic configuration of 24Cr is
A[Ar]3d54s1B[Ar]3d44s2C[Ar]3d34s24p1D[Ar]3d64s0
The ground state electronic configuration of chromium is against
View Solutionthe ground state electronic configuration of chromium can be written as
View SolutionThe ground state electronic configuration of bismuth (Z=83) is
View SolutionThe correct ground state electronic configuration of chromium atom is :
View SolutionWhich one is the correct ground state electronic configuration :
View Solution

