The relationship which describes the variation of vapour pressure with temperature is called
Hess's law
Arrhenius equation
Kirchoff's law
Clausis-Clapeyron equation
The correct Answer is:D
Factual question
Similar Questions
Knowledge Check
The curve showing the variation of adorpton with pressure at constant temperature is called :
Aan isosthermBadsorpton isothermCabsorption isothermDall of theseIf heat energy is given to an ideal gas at constant pressure, then select the graph which best represents the variation of VT with temperature (T).
A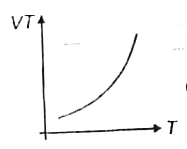 B
B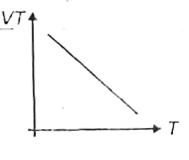 C
C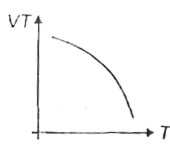 D
D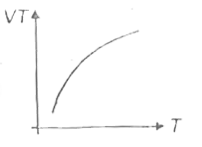
The curve showing the variation of pressure with temperature for a given amount of adsorption is called
Aadsorption isobarBadsorption isothermCadsorption isostereDadsorption isochore.
The variation of vapour pressure with temperature is quantitatively studied by ……………equation.
View SolutionDefination OF Solution || Vapour Pressure OF a Liquid: Evaporation & Condensation || Variation OF Vapour Pressure With Temperature, Boiling Point
View Solution(a) Derive a relationship between relative lowering of vapour pressure and mole fraction of the volatile liquid. (b) (i) Benzoic acid completely dimerises in benzene. What will be vapour pressure of a solution containing 61 g of benzoic acid per 500 g of benzene when the vapour pressure of pure benzene at the temperature of experiment is 66.6 torr ? (ii) What would have been the vapour pressure in the absence of dimerisation ? (iii) Derive a relationship between mole fraction and vapour pressure of a component of an ideal solution in the liquid phase and vapour phase.
View SolutionThe curveshowing the variation of pressure with temperature for a given amount of adsorption is called :
View SolutionThe curve showing the variation of pressure with temperature for a given amount of adsoption is called
View Solution

