Real gases deviated from ideal behaviour due to the following two faulty assumptions of kinetic theory of gases :
(i) Actual volume occupied by the gas molecule is negligible as compared to the total volume of the gases
(ii) Forces of attraction and repulsion among gas molecules are negligible
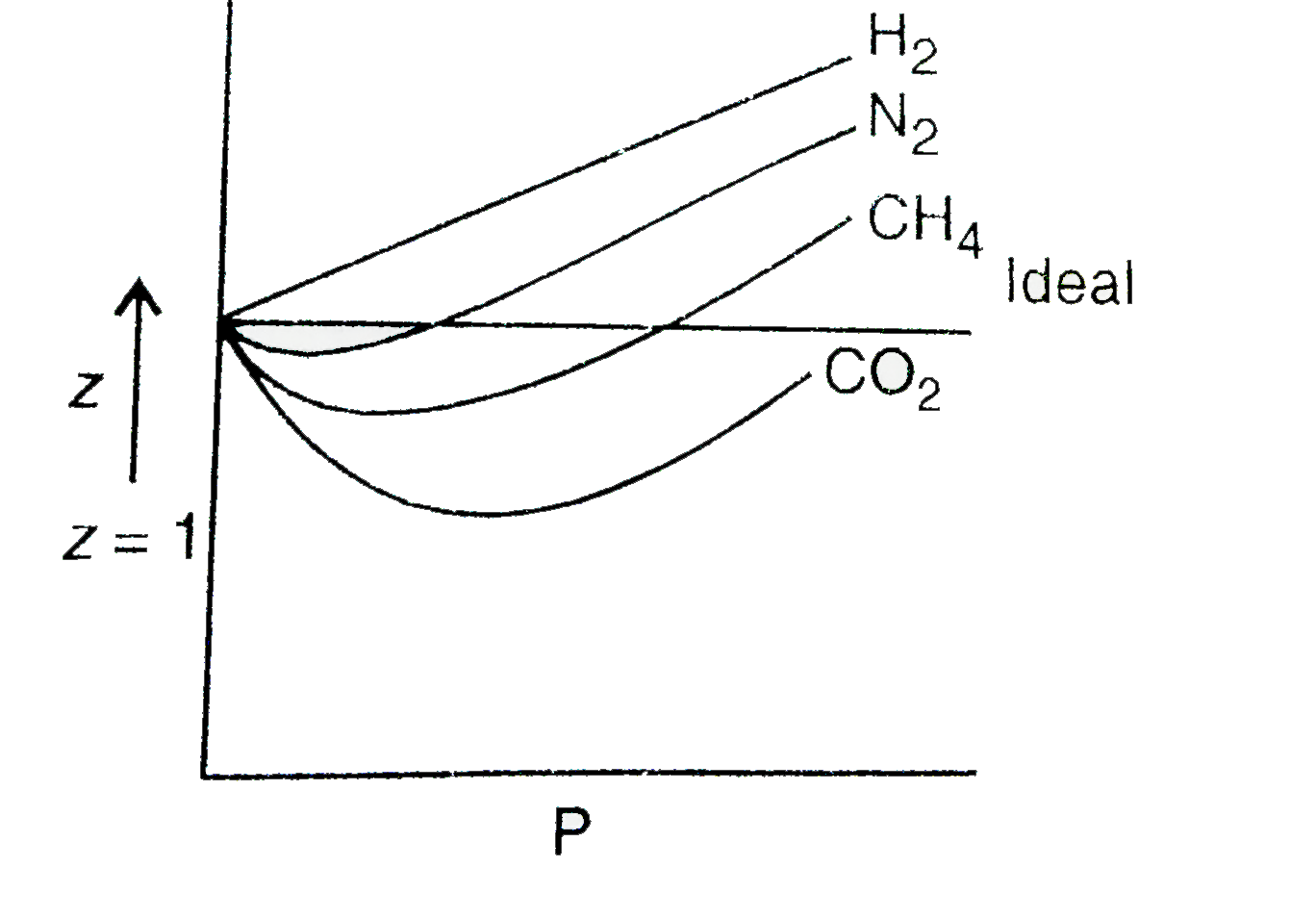
To explain the extent of deviation of the real gas from ideal behaviour in terms of compressibility or compression factor (z), which is the function of pressure and temperature for real gases z=P0V0nRT For ideal gases z=1. for real gases either z>1 or z>1.
When z>1, then it is less compressible because force of repulsion dominates over force of attraction. When z<1, force of attraction dominates over the repulsion and it is more compressible.
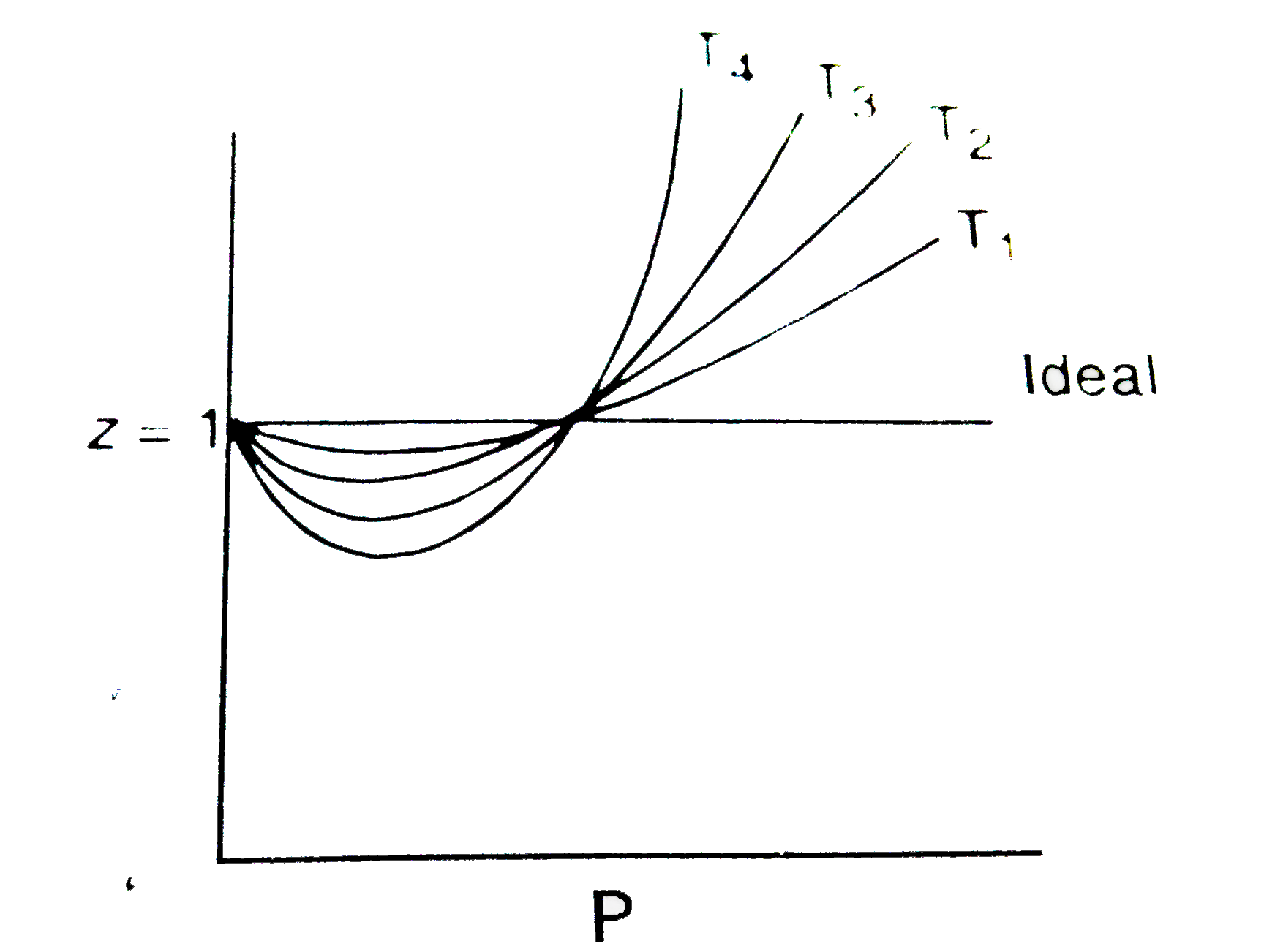
Graph in between z and P is shown below on increasing the temperature, z increases and approaches to unity. Graph between z and p at different temperature are as under
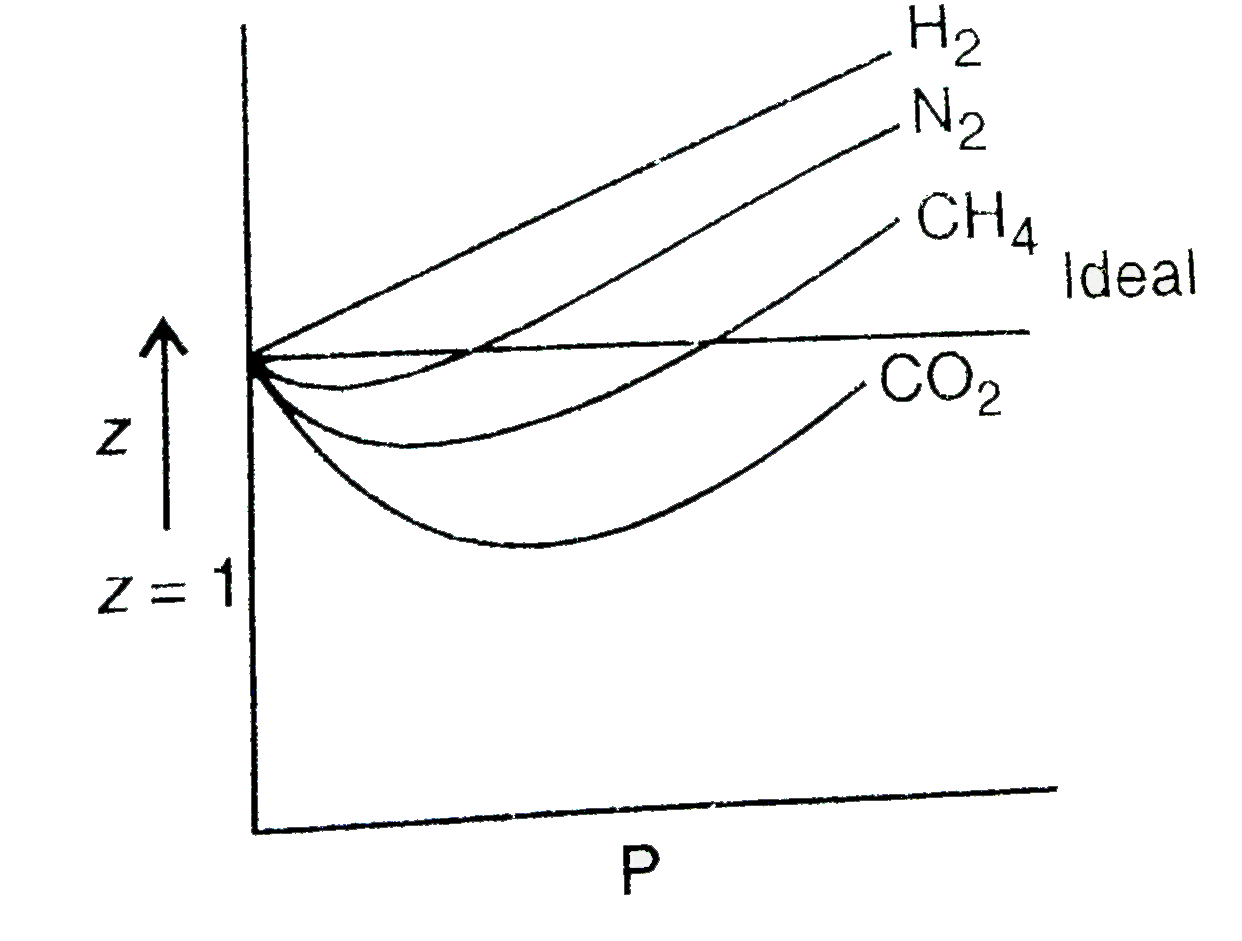
Answer the following questions on the basis of above write up :
Which of the following statements is correct ?
The compressibility factor for ideal gases is dependent of temperature and pressure
(dzdP)T for real gas is independent of pressure
(dzdP)T for all real gases have same value
(dzdP)T for different real gases have different values
The correct Answer is:A
Similar Questions
Comment on the statements :
(i) Molecular volume in a gas can be neglected as compared to the total volume of the gas.
(ii) Forces of attraction and repulsion among the gas molecules are negligible.
View SolutionReal gases deviate from ideal behaviour at low temperature and high pressure.
View Solution
Knowledge Check
Real gases deviated from ideal behaviour due to the following two faulty assumptions of kinetic theory of gases :
(i) Actual volume occupied by the gas molecule is negligible as compared to the total volume of the gases
(ii) Forces of attraction and repulsion among gas molecules are negligible
To explain the extent of deviation of the real gas from ideal behaviour in terms of compressibility or compression factor (z), which is the function of pressure and temperature for real gases z=P0V0nRT For ideal gases z=1. for real gases either z>1 or z>1.
When z>1, then it is less compressible because force of repulsion dominates over force of attraction. When z<1, force of attraction dominates over the repulsion and it is more compressible.
Graph in between z and P is shown below on increasing the temperature, z increases and approaches to unity. Graph between z and p at different temperature are as under
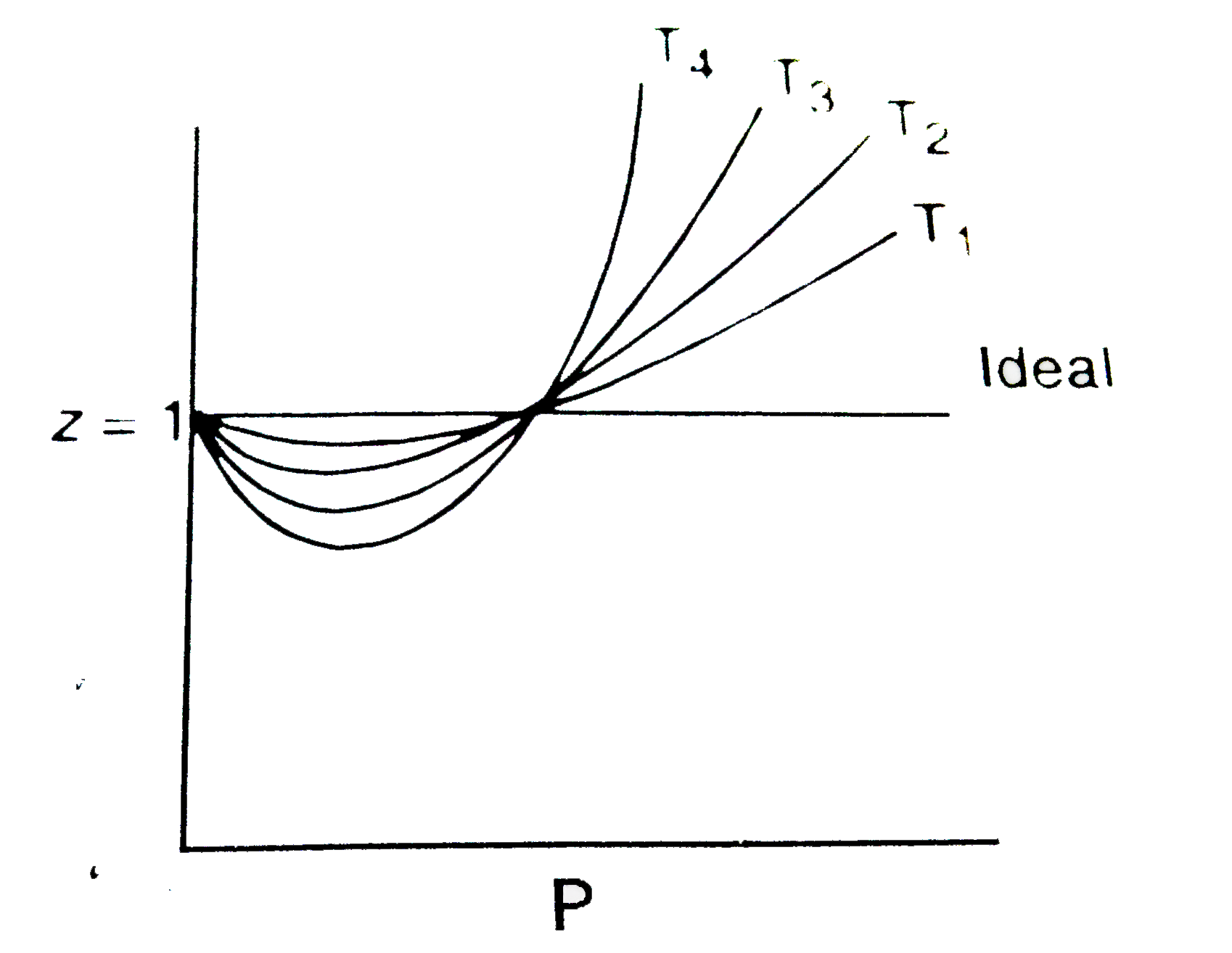
Answer the following questions on the basis of above write up :
Which of the following is the correct order of temperature shown in the above graph z vs P for the same gasAT4>T3>T2>T1BT1>T2>T3>T4CT1>T2>T4>T3DT3>T4>T2>T1Real gases deviated from ideal behaviour due to the following two faulty assumptions of kinetic theory of gases :
(i) Actual volume occupied by the gas molecule is negligible as compared to the total volume of the gases
(ii) Forces of attraction and repulsion among gas molecules are negligible
To explain the extent of deviation of the real gas from ideal behaviour in terms of compressibility or compression factor (z), which is the function of pressure and temperature for real gases z=P0V0nRT For ideal gases z=1. for real gases either z>1 or z>1.
When z>1, then it is less compressible because force of repulsion dominates over force of attraction. When z<1, force of attraction dominates over the repulsion and it is more compressible.
Graph in between z and P is shown below on increasing the temperature, z increases and approaches to unity. Graph between z and p at different temperature are as under
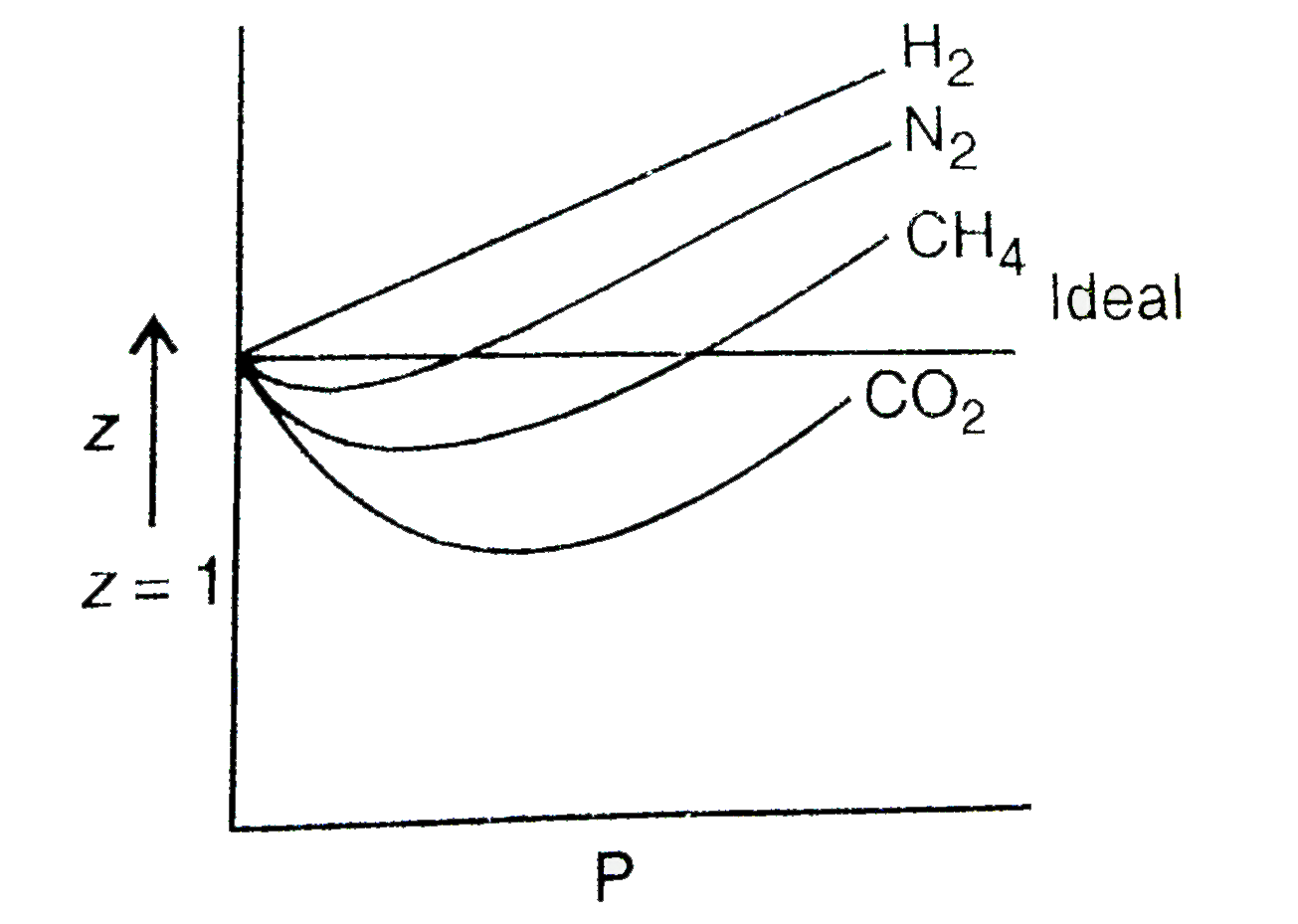

Answer the following questions on the basis of above write up :
What is correct increasing order of liquefibility of the gases shown in the above graph ?AH2<N2<CH4<CO2BCO2<CH4<N2<H2CH2<CH4<N2<CO2DCH4<H2<N2<CO2Real gases show deviation from ideal behaviour at low temperature and high pressure.
Atemperature is low and pressure is highBtemperature si high and Pressure is lowCboth temperature and pressure are lowDboth temperature and pressure are high
Under which of the following conditions applied together, a gas deviates most from the ideal behaviour ?
View SolutionWhat are ideal and real gases? What is the cause of deviation of real gases from the ideal gas behaviour ?
View SolutionReal gases deviate from an ideal behaviour because gas molecules
View SolutionGases deviate from the ideal gas behaviour because their molecules
View SolutionGases deviate from the ideal gas behaviour because their molecules
View Solution

