In a solid 'AB' having NaCl structure atoms occupy the corners of the cube unit cell. If all the face centred atoms along one of the axis are removed then the resultant stoichiometry of the solid is
AB2
A2B
A4B3
A3B4
The correct Answer is:C
In one NaCl type unit cell of solid B−are present at corners and face centred and A+ are present at edge centres and body centre. Thus, a unit cell will contain 4 A+and4B−ions. In this unit two face centred ions B−lies along one axis and are removed.
Contribution of two fece centred B−ions
=2×1/2=1
In the resultant unit cell,
∴No.of A+ ions present per unit cell =4
∴No. of B−ions present per unit cell
=4-1=3resultant.
∴Stoichiometry=A4B3
Topper's Solved these Questions
- STATES OF MATTER (SOLID STATE CHEMISTRY)BOOK - DINESH PUBLICATIONCHAPTER - STATES OF MATTER (SOLID STATE CHEMISTRY)EXERCISE - LINKED COMPREHENSION TYPE MCQs
12 Videos
- STATES OF MATTER (SOLID STATE CHEMISTRY)BOOK - DINESH PUBLICATIONCHAPTER - STATES OF MATTER (SOLID STATE CHEMISTRY)EXERCISE - MATRIX MATCH TYPE MCQs
4 Videos
- STATES OF MATTER (SOLID STATE CHEMISTRY)BOOK - DINESH PUBLICATIONCHAPTER - STATES OF MATTER (SOLID STATE CHEMISTRY)EXERCISE - REVISION QUESTIONS FROM COMPETITIVE EXAMS
104 Videos
- STATE OF MATTER (GASEOUS AND LIQUID STATE)BOOK - DINESH PUBLICATIONCHAPTER - STATE OF MATTER (GASEOUS AND LIQUID STATE)EXERCISE - Ulitmate Preparatory package
20 Videos
- STATES OF MATTER : GASES AND LIQUIDSBOOK - DINESH PUBLICATIONCHAPTER - STATES OF MATTER : GASES AND LIQUIDSEXERCISE - Statement Type Question
5 Videos
Similar Questions
In hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagon and three atoms are sandwiched in between them. A space filling model of this structure, called hexagonal close packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical sphere as closely as possible. Three spheres are then placed over the first layer so that they touch other and represent the second layer. Each one of the three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be same
The number of atoms in this HCP unit cell is
View SolutionStatements in Column I are labelled as A, B, C and D whereas the statements in Column II are labelled as p, q, r and s. The answers to these questions are to be appropriately bubbled as illustrated below in the following example. If the correct matches are A-p, A-s, B-q, B-r, C-p, C-q and D-p, their corretly labelled 4times 4 matrix should look like :
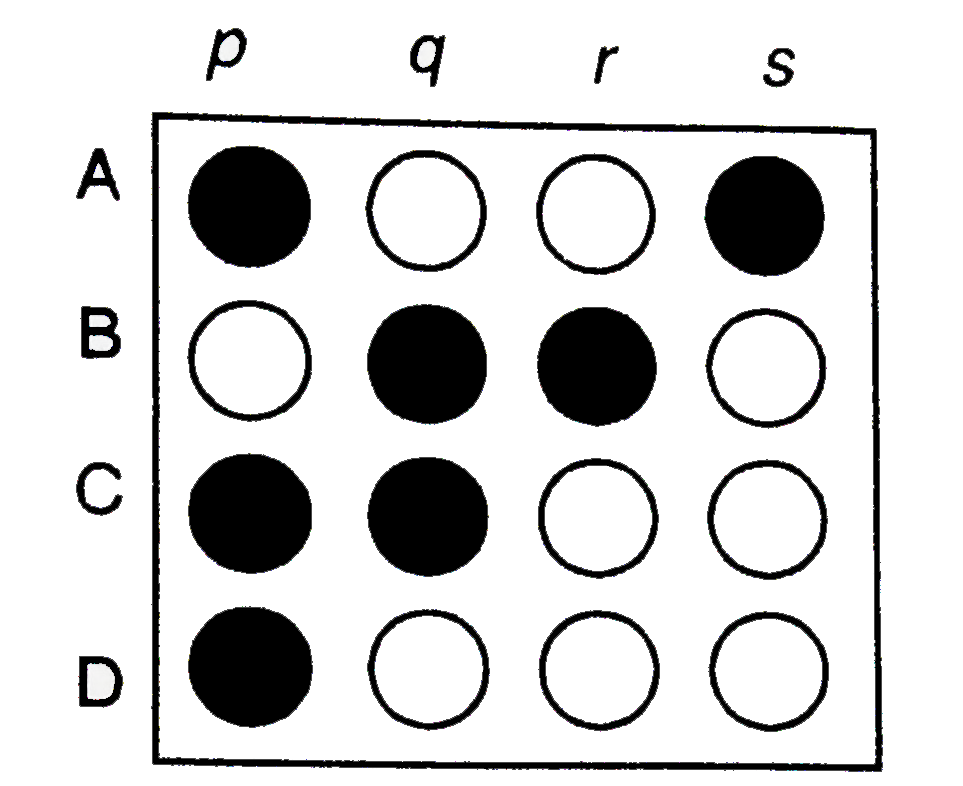
Column-IColumn-II(A)NaClp.Crystalline(B)p-type semiconductorq.Doping(C)n-type semiconductorr.holes are responsible for movement of current(D)Graphites.electrons are responsible for movement of current
View SolutionWhich of the following is hcp crystal structure ?
View SolutionIf atomic mass of hydrogen on a hypothetical scale is choosen to be 10.0000, what is the molecular mass of a gas which has a vapour density of 22 ?
View SolutionStatement-1. Considering van der Waals' equation of state of NH3 and N2
[P+aV2][V−b]=RT
The value of van der Waals' constant for NH3 is more than tht for N2
Statement-2. Ammonia has lower molecular mass than N2.
View SolutionIn a solid AB having the NaCl structure, A atom occupies the corners of the cubic unit cell. If all the face-centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
View SolutionIn a solid AB having the NaCl structure, A atom occupies the corners of the cubic unit cell. If all the face-centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
View SolutionIn a solid 'AB' having NaCl structure , 'A' atoms occupy the corners of the cubic unit cell. If cell the face-centred atoms along one of the axes are removed , then the resultant stoichiometry of the solid is
View SolutionIn a solid AB having NaCl structure 'A' atoms occupy the corners & face centre of the cubic unit cell. If all the face centered atoms along one of the axes are removed, then the resultant stoichiomery of the solid is
View SolutionIn a solid 'AB' having rock salt arrangement ( NaCl structure), 'A' atoms occupy the corners and face center of the cubic unit cell. 'B' atoms occupies all octahedral voids of the unit cell. If all the face - centered atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
View SolutionIn a solid AB having the NaCl structure, A atom occupies the corners of the cubic unit cell. If all the face-centered atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
View SolutionIn a solid 'AB' having NaCl structure, 'A' atoms occupy the corners of the cubic unit cell. If all the face-centred atoms along one of the axes are removed,then the resultant stiochiometry of the solid is
View SolutionIn a solid AB having the NaCZ structure. ‘A’ atoms occupy the corners of the cubic unit cell. If all the edge-centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
View SolutionIn a solid AB having the NaCZ structure. ‘A’ atoms occupy the corners of the cubic unit cell. If all the edge-centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
View SolutionIn a solid .AB. hasving the NaCl structure, .A. atoms occupy the corners of the cubic unit cell. If the face - centrad atoms along one of the axis are removed, then the resultant stoichiometry of ther solid is
View Solution

