For a reaction SO2(g)+1/2O2(g)⇔SO3(g) ltbr The valve of KPKC is equal to
1
RT
(RT)1/2
(RT)−1/2
The correct Answer is:D
Step by step video, text & image solution for For a reaction SO_(2(g))+1//2O_(2(g))hArrSO_(3(g)) ltbr The valve of (K_(P))/(K_(C)) is equal to by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:INORGANIC CHEMISTRY
Explore 300 Videos
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 483 Videos
- Chemical EquilibriumBook:ALLENChapter:Chemical EquilibriumExercise:All Questions
Explore 24 Videos
- ELECTROCHEMISTRYBook:ALLENChapter:ELECTROCHEMISTRYExercise:EXERCISE -05 [B]
Explore 38 Videos
Similar Questions
If atomic number 117,120 are discovered then their blocks will be
View SolutionIUPAC Name of following compound is :
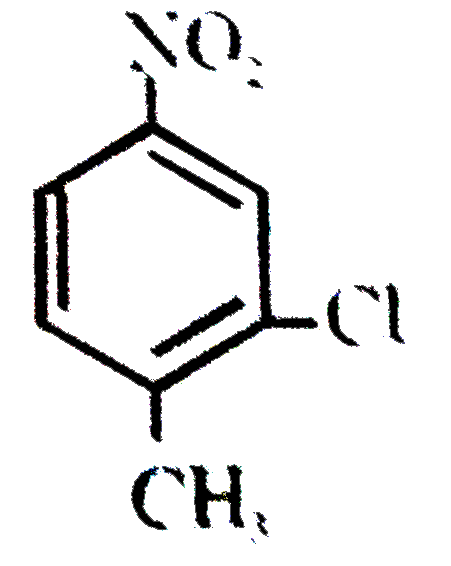
View SolutionIf the forward rate constant of reversible reaction is 0.16 and backward rate constant is 4×104 , then the equilibrum constant will be .
View SolutionFind the solubility product of a saturated solution of Ag2CrO4 in water at 298K, if the EMF of the cell :
Ag∣∣Ag⊕(satAg2CrO4sol)∣∣|Ag(0.1M)|Agis0.164V at 298K.
View SolutionThe equilibrium constant for the given reaction:
SO3(g)⇔SO2(g)+1/2O2(g), (Kc=4.9×10−2)
The value of Kc for the reaction:
2SO2(g)+O2(g)⇔2SO3(g), will be :
View SolutionFor the reaction, SO2(g)+12O2(g)⇔SO3(g), If Kp=Kc(RT)x where the symbols have usual meaning then, the value of x is (assuming ideality).
View SolutionFor the reaction, SO_(2)(g)+1/2O_(2)(g)hArrSO_(3)(g) if K_(p)=K_(C)(RT)^(x) where, the symbols have usual meaning, then the value of x is (assuming ideality)
View SolutionFor the reaction SO2(g)+12O2(g)⇔SO3(g), if Kc=Kp(RT)X then the value of X is
View SolutionFor a reaction,
SO2(g)+1/2O2(g)→SO3(g) if Kp=1.7×1012 at 20∘C and 1 atm pressure , then value of Kc is
View SolutionFor the reaction CO(g)+CI2(g)⇔COCI2(g) the valve of KcKp is euual to :-
View SolutionFor the reaction, SO_(2)(g)+1//2O_(2)(g)hArrSO_(3)(g) DeltaH_(298)^(@)=-98.32KJ//mol e,DeltaS_(298)^(@)=-95.0J//mol e-K . Find the K_(p) for this reaction at 298K.
View SolutionTwo gaseous equlibriua SO2(g)+12O2(g)⇔SO3(g)and2SO3(g)⇔2SO2(g)+O2(g) have equlibrium constants K1andK2 respectively at 298K. Which of the following relationship between K1andK2 is correct
View SolutionFor the reaction CO(g)+12O2(g)⇔CO2(g),KpKc is equivalent to
View SolutionCO(g)+1/2O2(g)<⇒CO2(g) अभिक्रिया में Kp/Kc है:
View SolutionAt 500K,Kp value for the reaction
2SO2(g)+O2(g)⇔2SO3(g) is 2.5×1010.
Find the value of Kp for each of followign reactions at the same temperature.
a. SO2(g)+1/2O2(g)⇔SO3(g)
b. SO3(g)⇔SO2(g)+1/2O2(g)
c. 3SO2(g)+3/2O2(g)⇔3SO3(g)
View Solution
