A methane +H2 mixture contains 10g methane and 5g Hydrogen. If the pressure of the mixture is 30 atm. What is the partial pressure of H2 in moxture :-
6 atm
129 atm
24 atm
189 atm
The correct Answer is:C
PH2=nH2nH2+nCH4
PH2=5252+1016×30
Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:INORGANIC CHEMISTRY
Explore 300 Videos
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 483 Videos
- Chemical EquilibriumBook:ALLENChapter:Chemical EquilibriumExercise:All Questions
Explore 24 Videos
- ELECTROCHEMISTRYBook:ALLENChapter:ELECTROCHEMISTRYExercise:EXERCISE -05 [B]
Explore 38 Videos
Similar Questions
If atomic number 117,120 are discovered then their blocks will be
View SolutionIUPAC Name of following compound is :
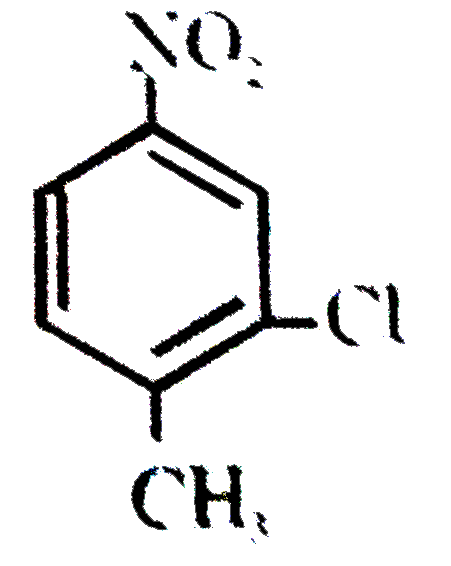
View SolutionIf the forward rate constant of reversible reaction is 0.16 and backward rate constant is 4×104 , then the equilibrum constant will be .
View SolutionFind the solubility product of a saturated solution of Ag2CrO4 in water at 298K, if the EMF of the cell :
Ag∣∣Ag⊕(satAg2CrO4sol)∣∣|Ag(0.1M)|Agis0.164V at 298K.
View SolutionAgaseous maxture was prepared by taking 3 mol of H_(2) and 1 mol of CO. If the total pressure of the mixture was found 2 atmosphere then partial pressure of hydrogen (H_(2)) in the mixture is :-
View SolutionA vessel contains equal masses of hydrogen, helium and methane. Find out the fractions of the partial pressures in the mixture.
View Solutionएक फ्लास्क में 2 ग्राम H2 तथा 16 ग्राम O2 का मिश्रण भरा है | यदि मिश्रण का सम्पूर्ण दाब 1 वायुमण्डल हो, तो ऑक्सीजन का आंशिक दाब क्या होगा ?
View SolutionIn a gas mixture of H2andHe, the partial pressure of H2 is half that of He. Find the mole fractions of H2 and He in the mixture.
View SolutionA mixture of O_(2) and H_(2) gases contains 20% of H_(2) gas. At a certain temperature, the total pressure of the mixture is found to be 1 bar. What is the partial pressure of O_(2) (in bar) in the mixture?
View SolutionA flask contains 3g H_(2) and 24g O_(2). The partial pressure of hydrogen in the mixture is
View Solution60 g of gaseous C_(2)H_(6) are mixed with 28 g of carbon monoxide. The pressure of the resulting gaseous mixture is 3 atm. The partial pressure in atm. Of C_(2)H_(6) in the mixture is
View SolutionA 2L flask contains 1.6 g of methane and 0.5g of hydrogen at 27^(@)C . Calculate the partial pressure of each gas the mixture and calculate the total pressure.
View SolutionIn a mixture of equal weights of H2 and He gases at pressure of "6" atm the partial pressure of helium is "
View Solutionएक पात्र में भरी हुई H2 और N2 गैस के मिश्रण का दाब 750 bar है। यदि मिश्रण में N2 का आंशिक दाब 150 bar हो मिश्रण में H2 और N2 के अणुओ का अनुपात क्या होगा।
View SolutionEqual mass of H_(2) , He and CH_(4) are mixed in empty container at 300 K, when total pressure is 2.6 atm The partial pressure of H_(2) in the mixture is
View SolutionIn a mixture of N_2 and CO_2 gases, the partial pressure of CO_(2) is 1.25 atm. The total pressure of the mixture is 5 atm. The mole fraction of N_(2) in the mixture is
View Solution
